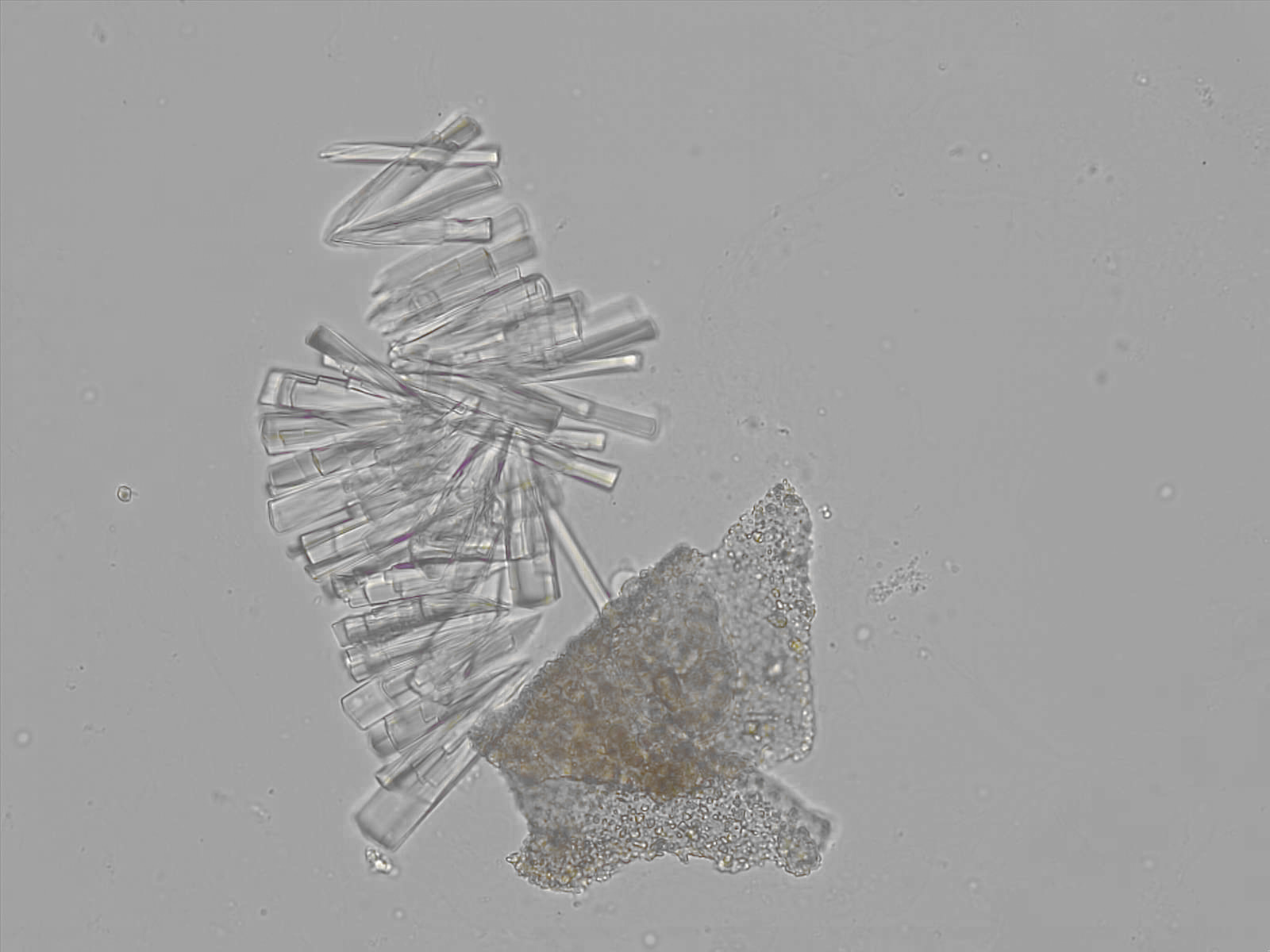
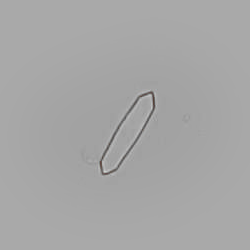
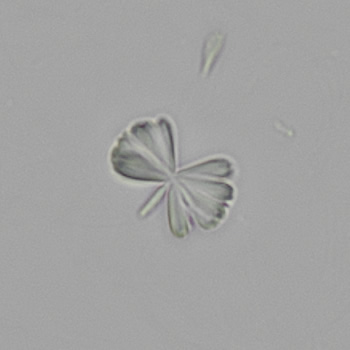
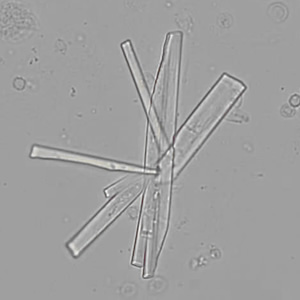
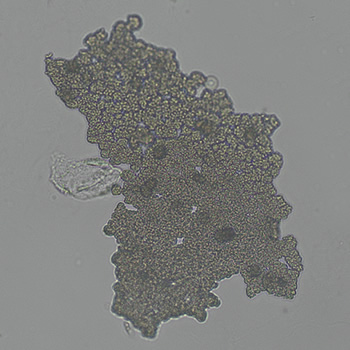
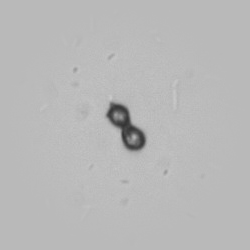
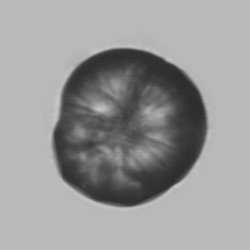
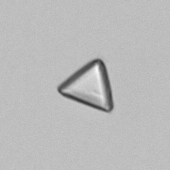
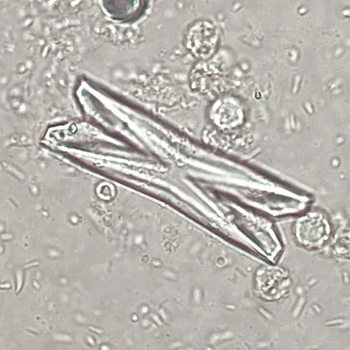
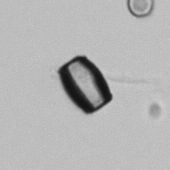
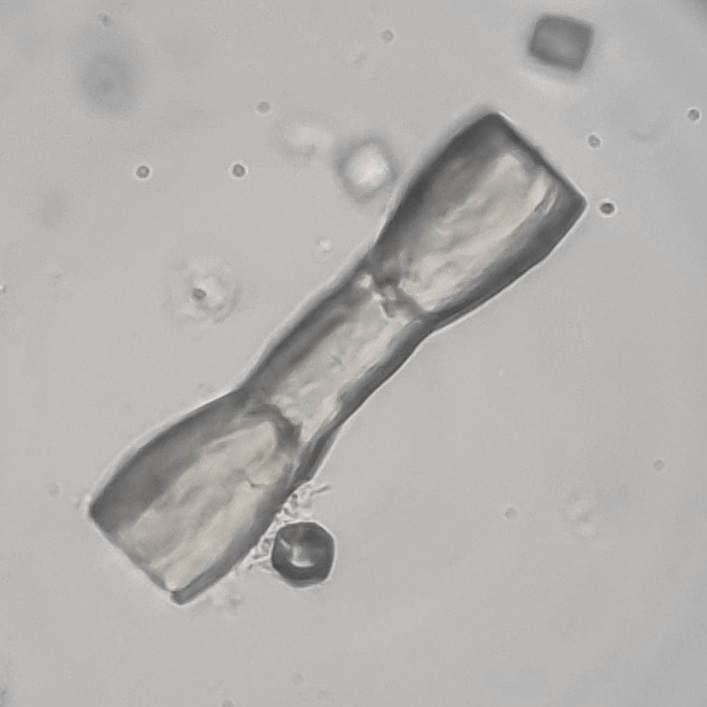
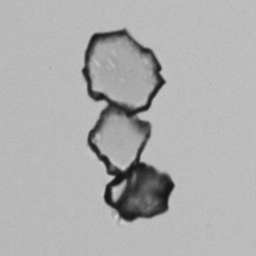
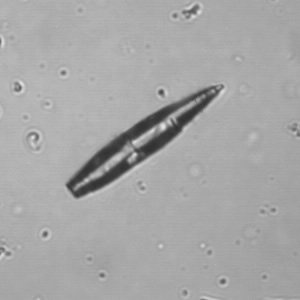
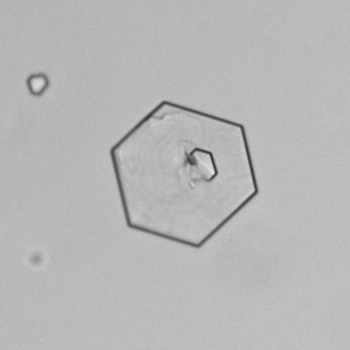
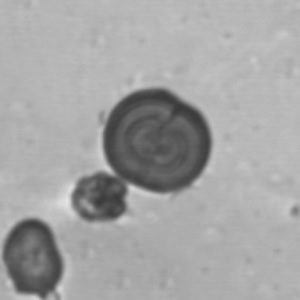
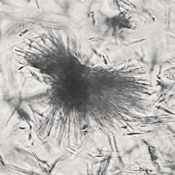
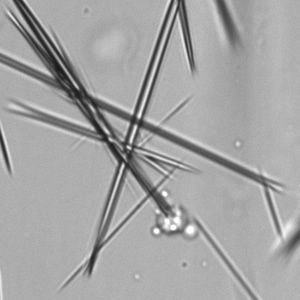
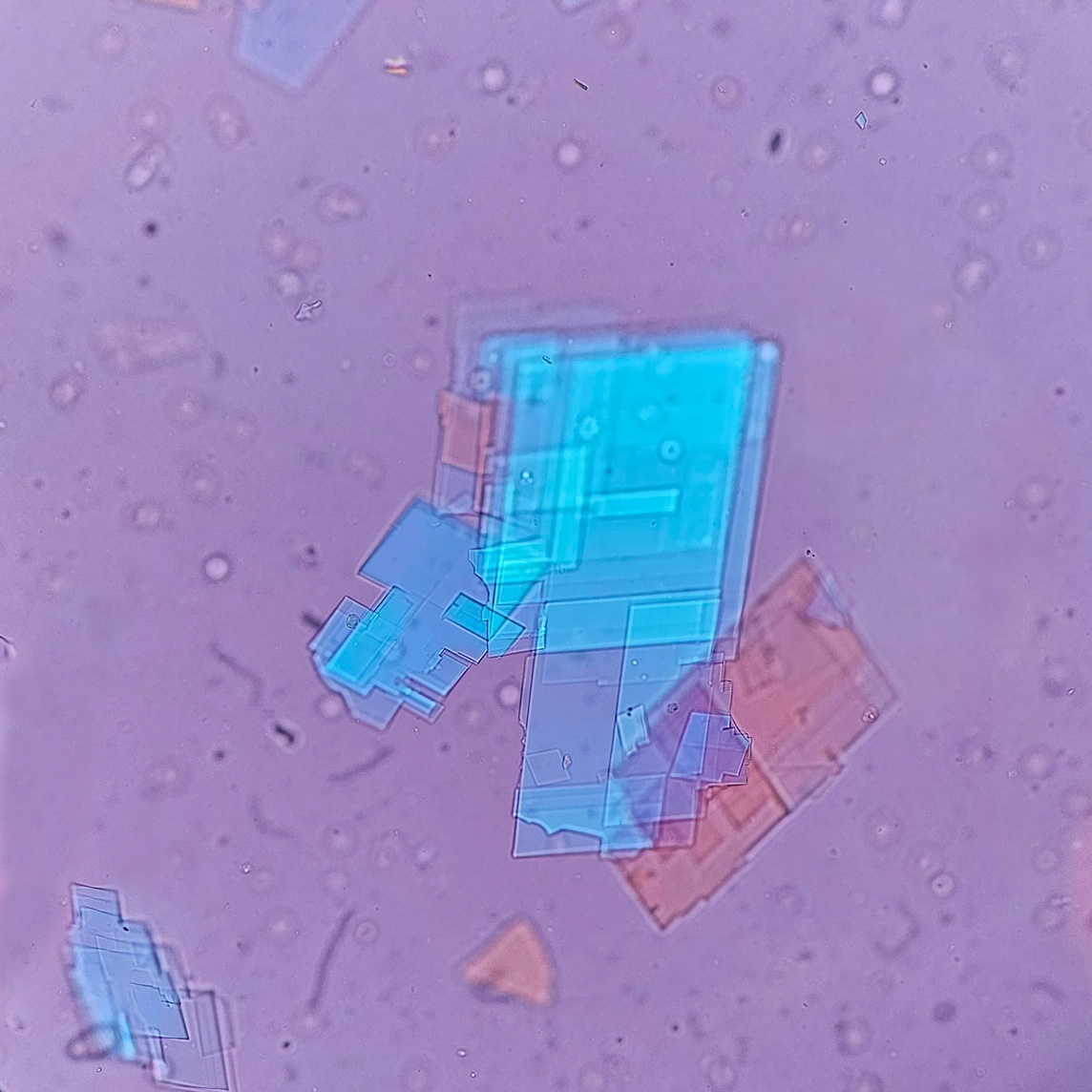
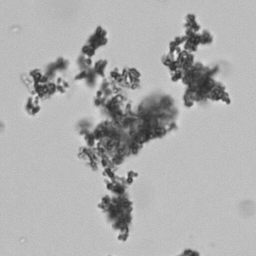
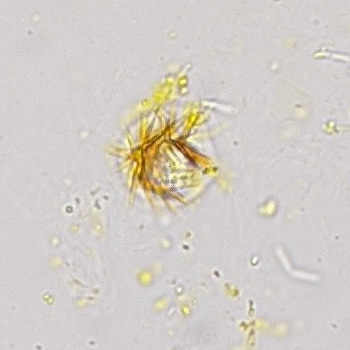
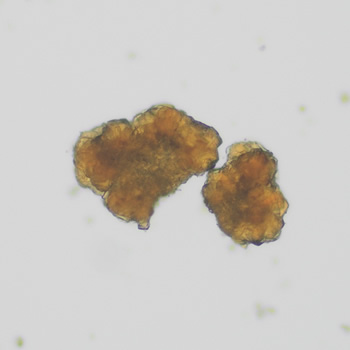
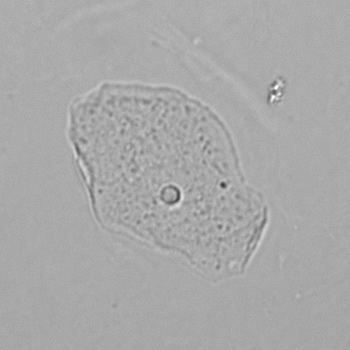
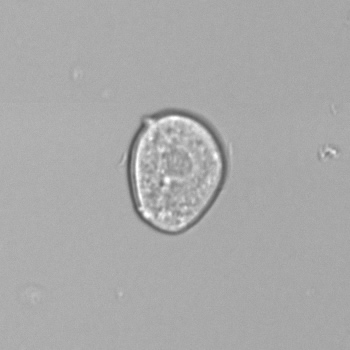
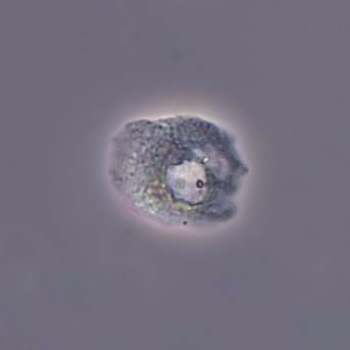
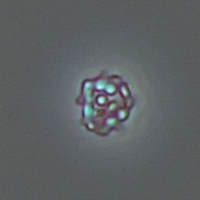
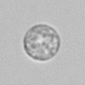
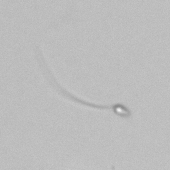
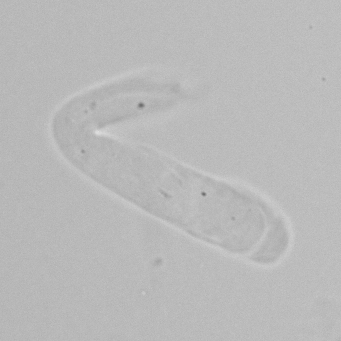
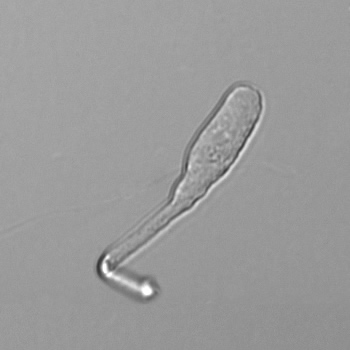
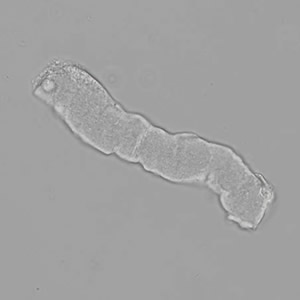
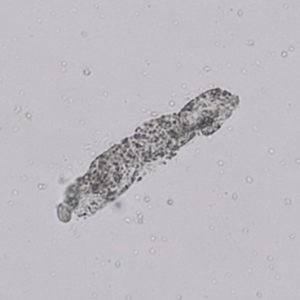
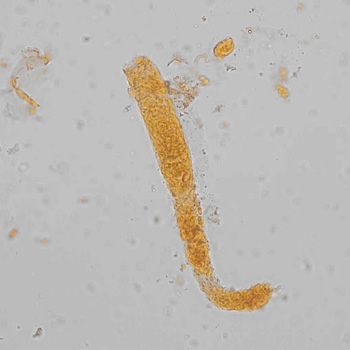
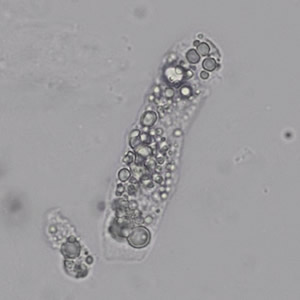
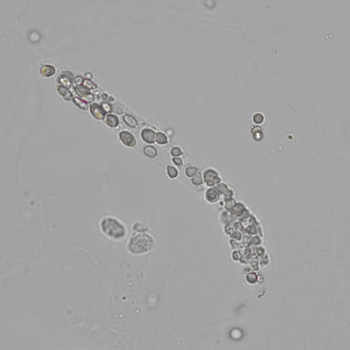
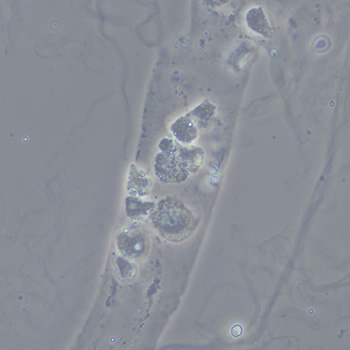
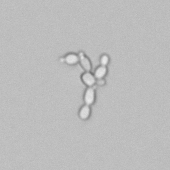
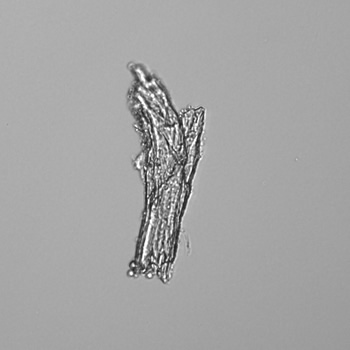
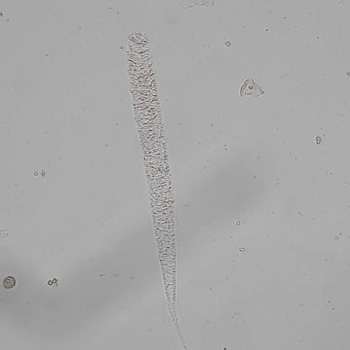
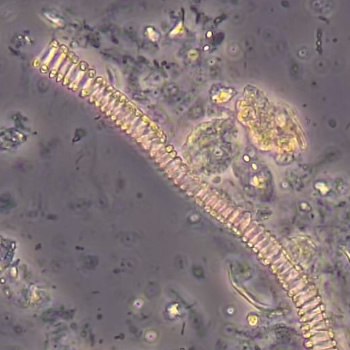
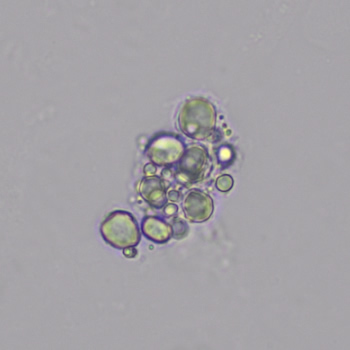
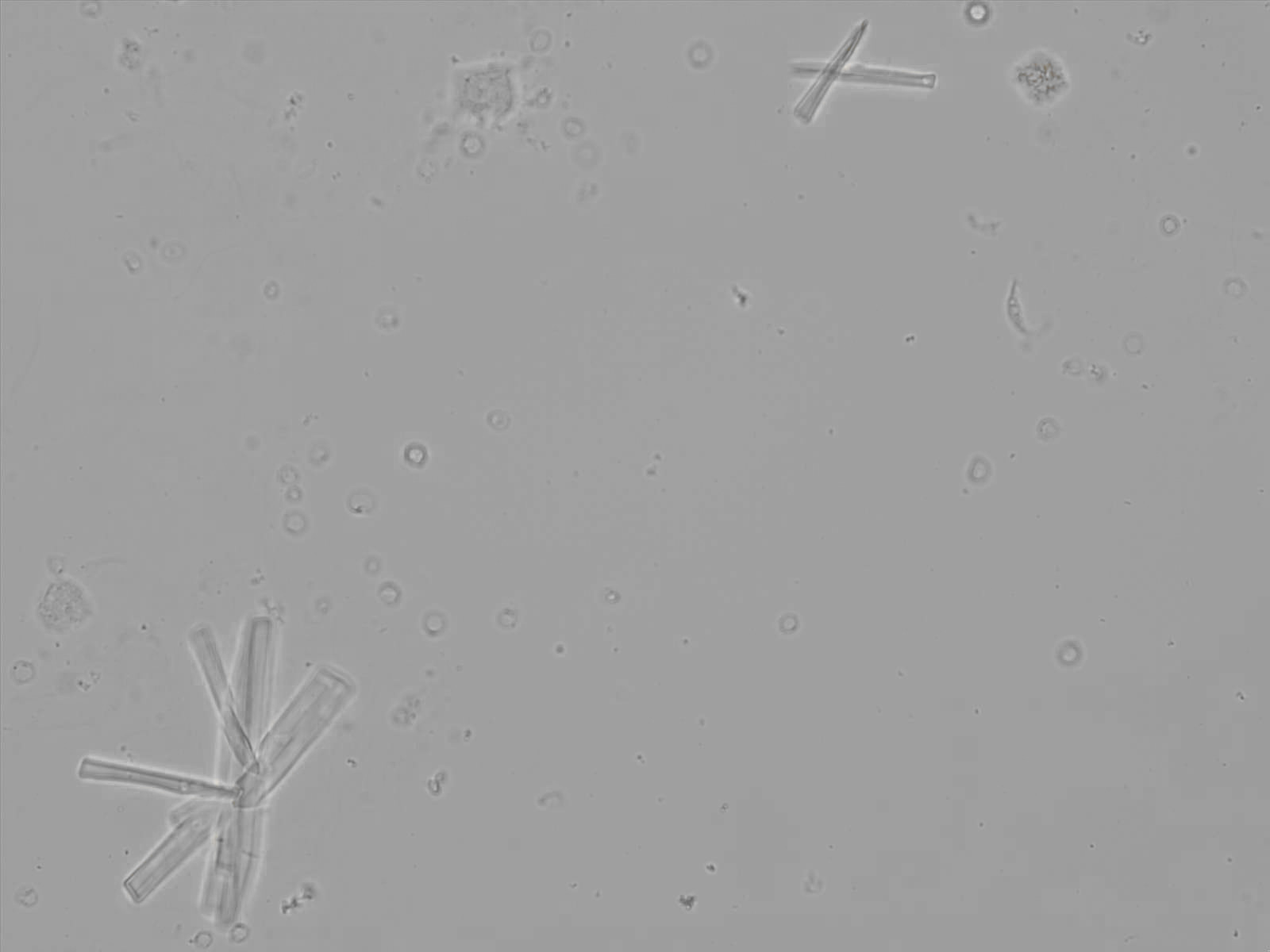
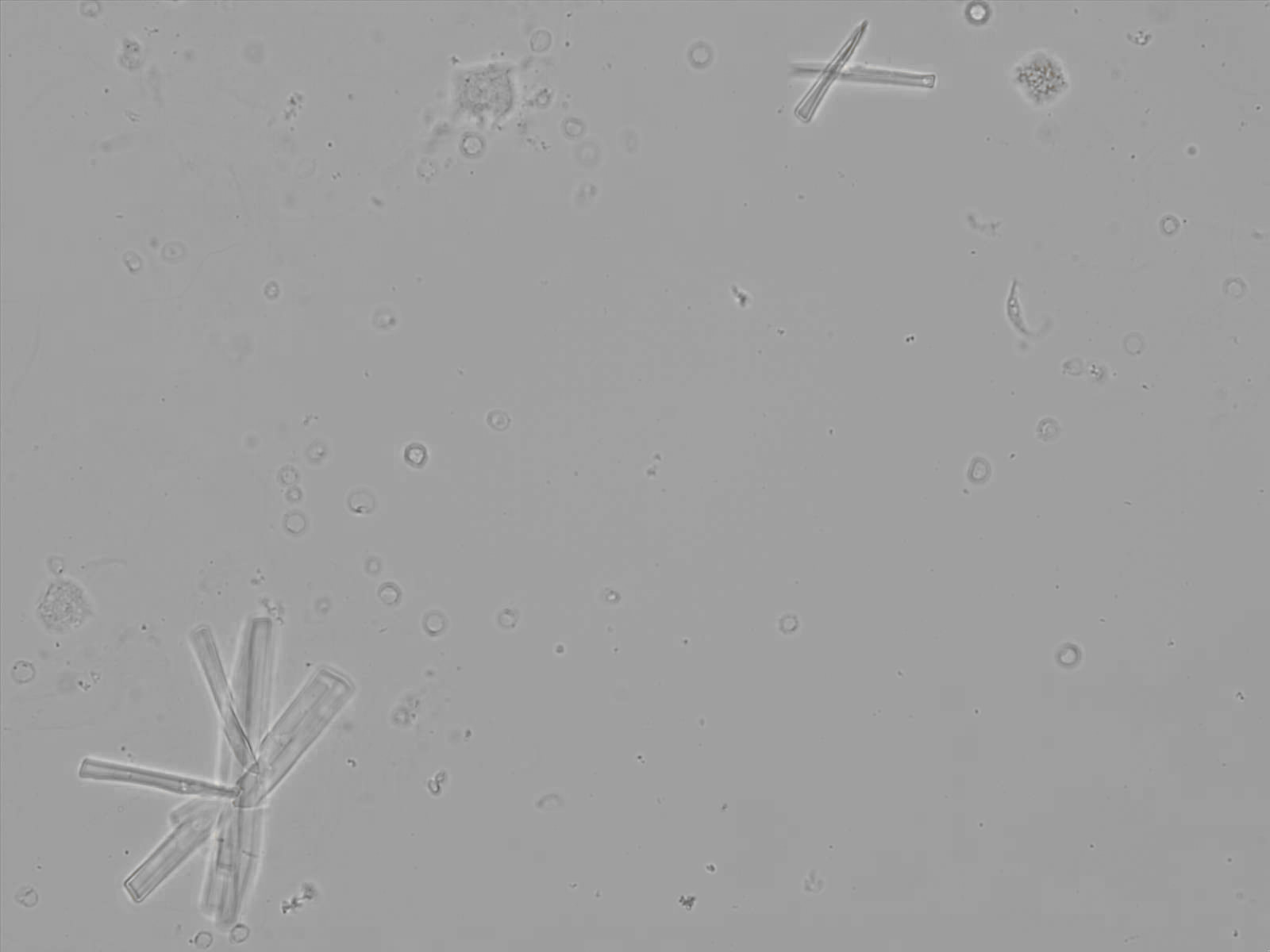
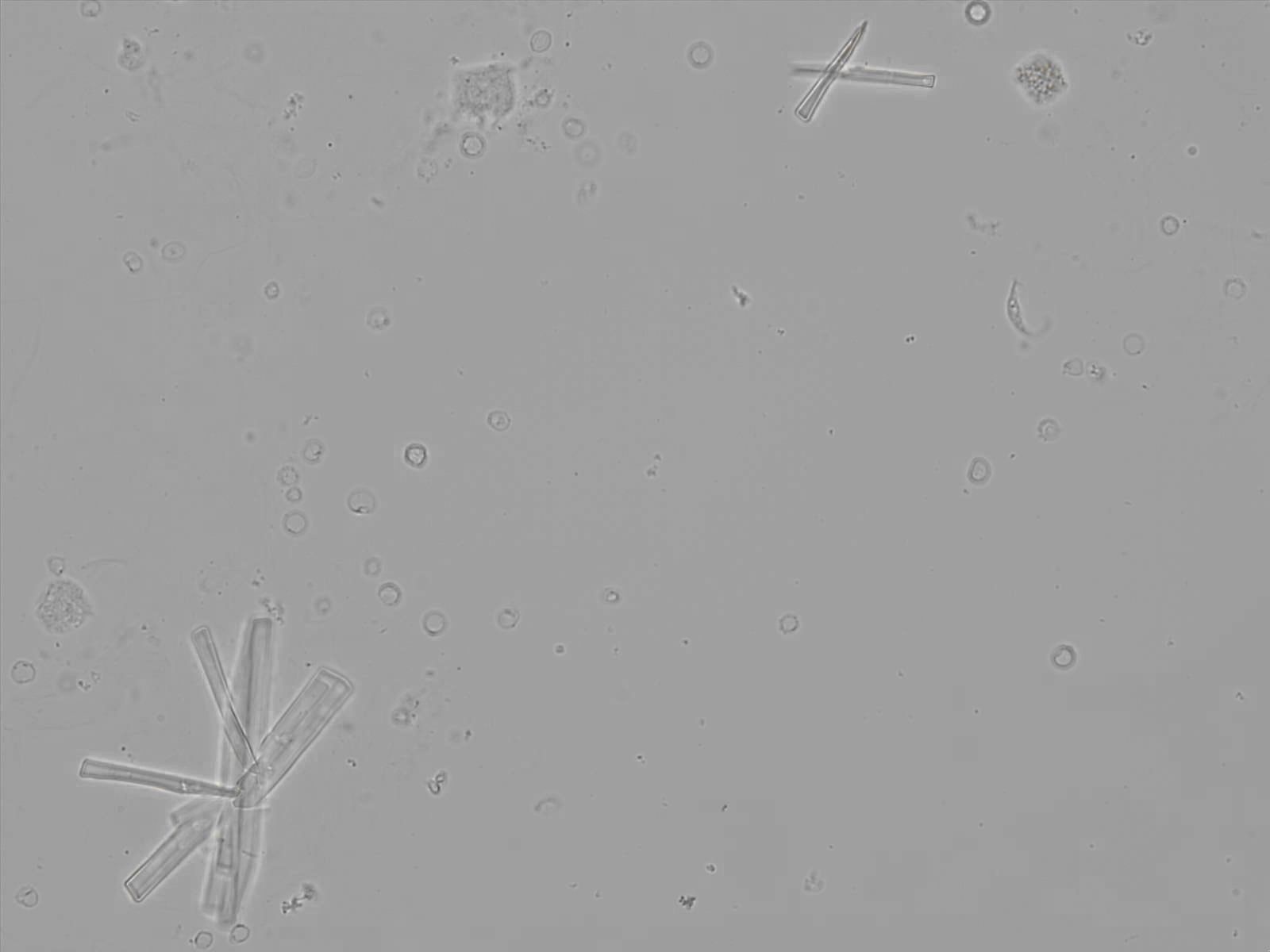
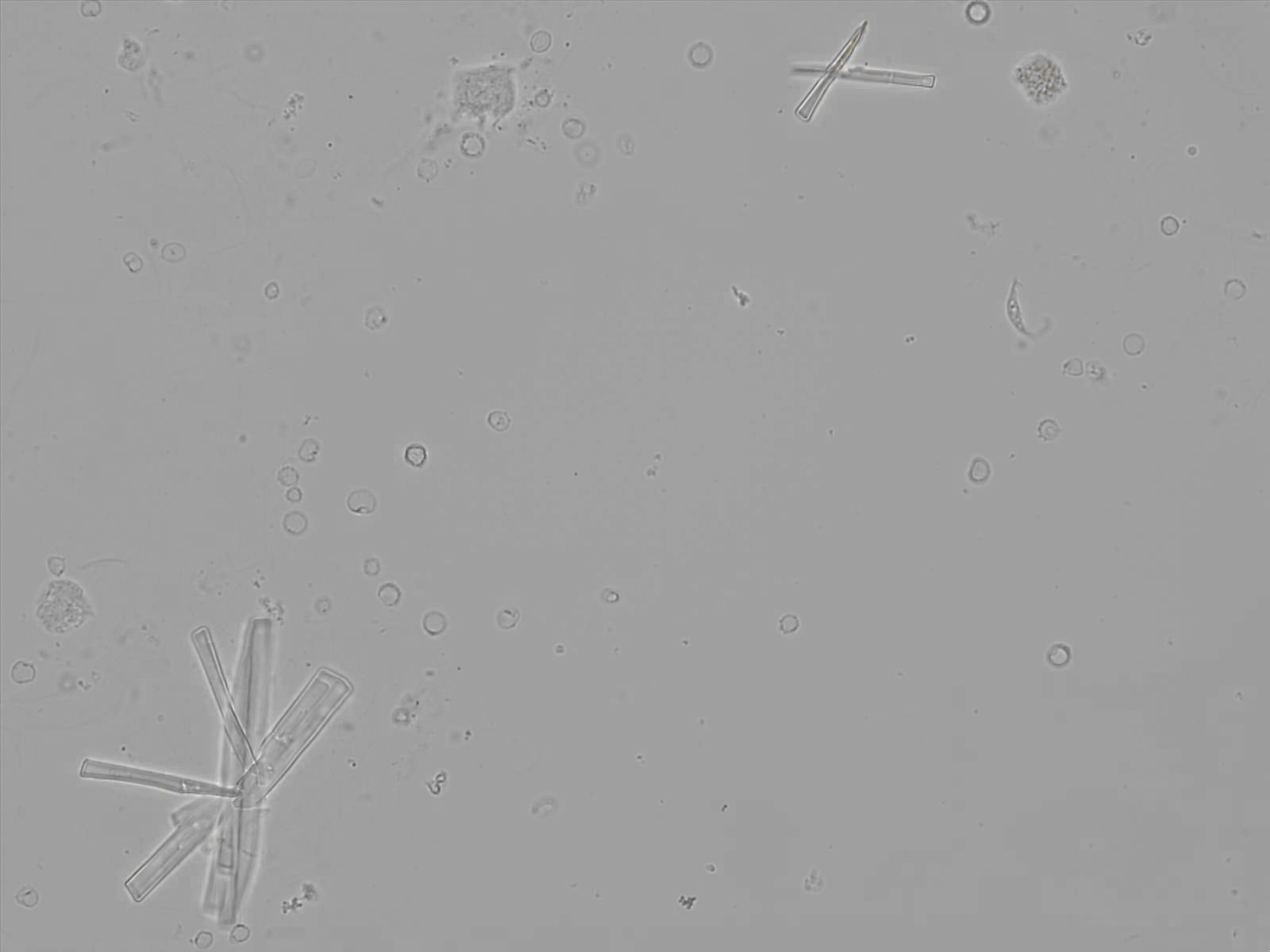
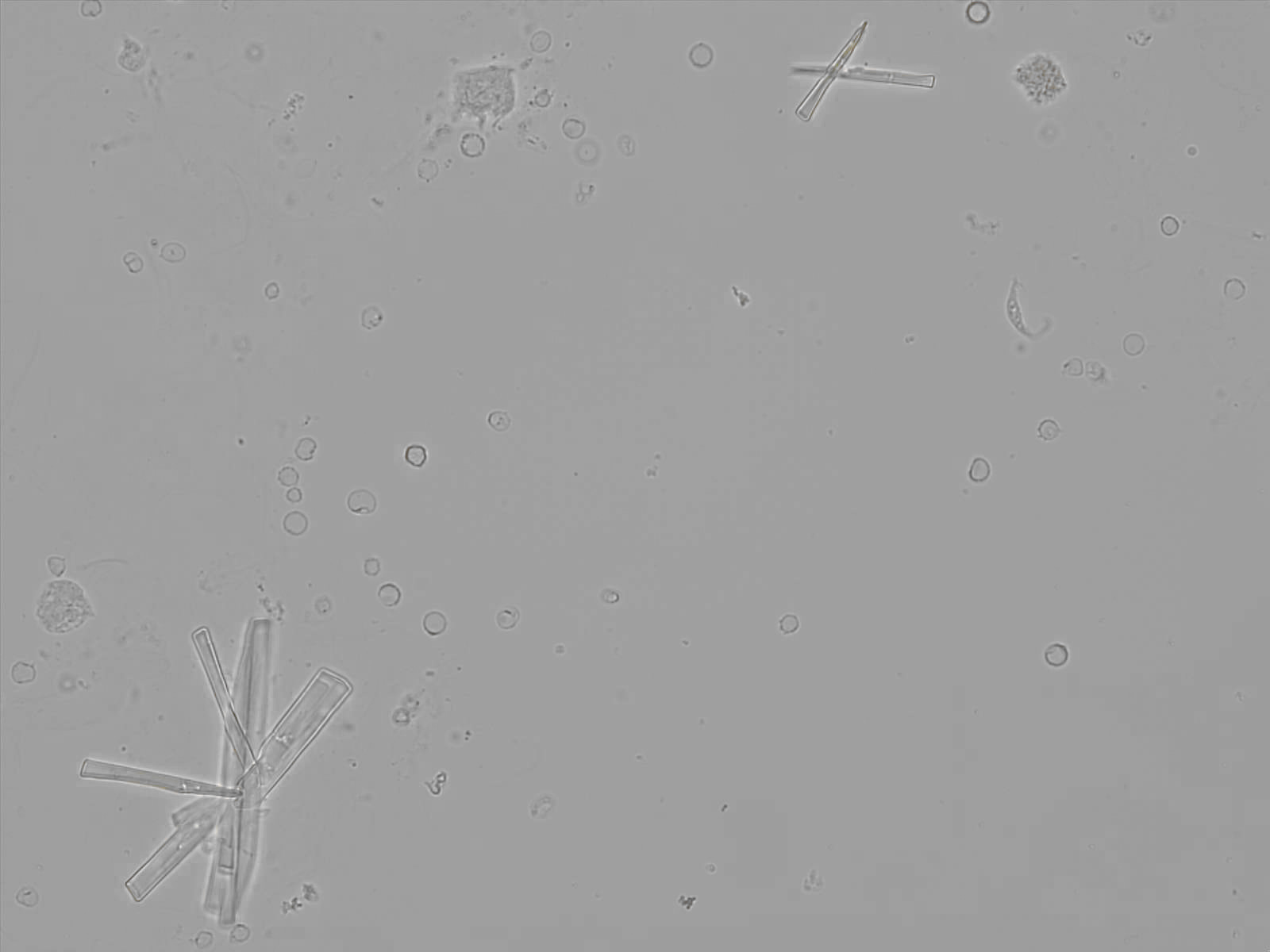
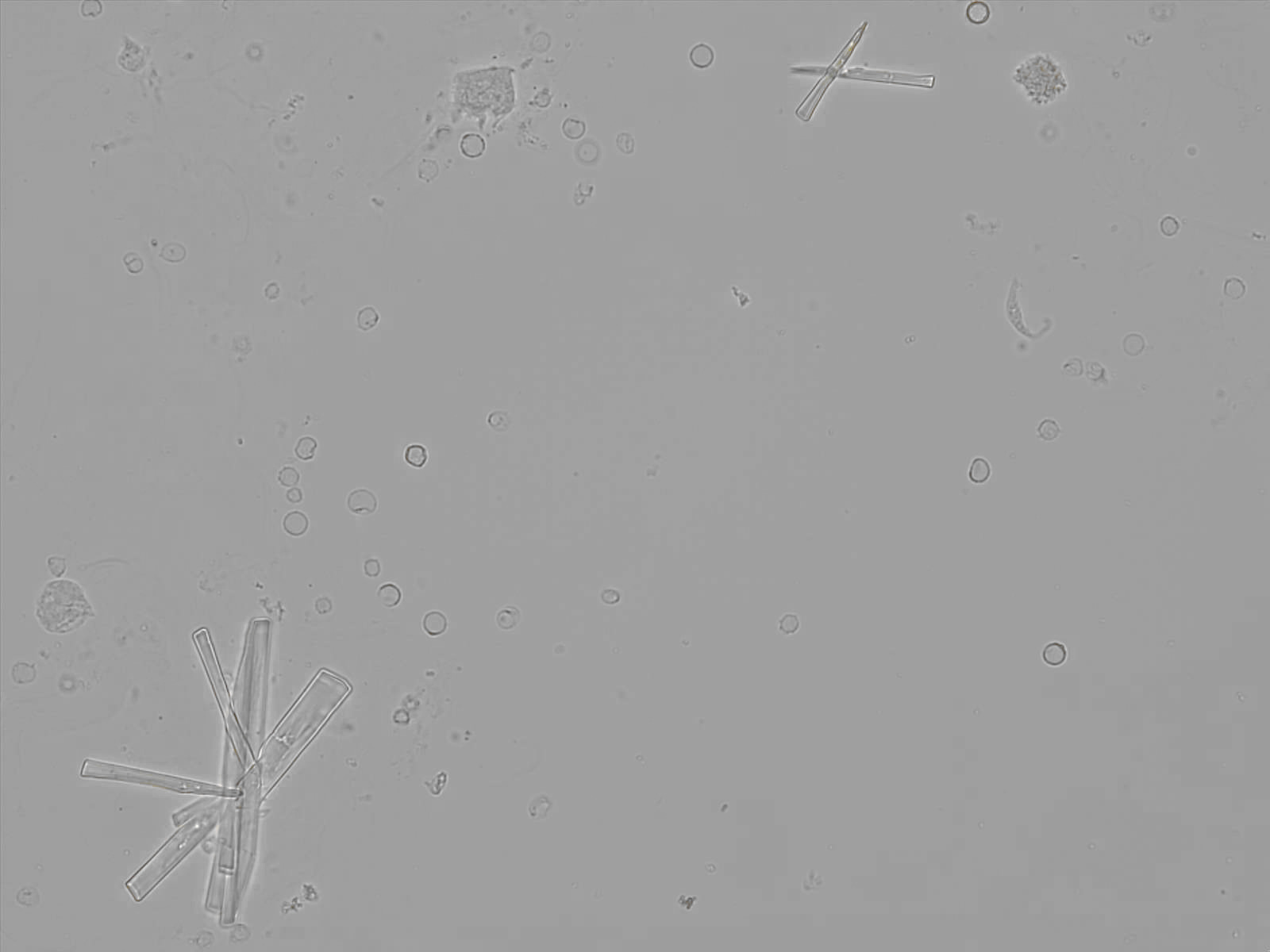
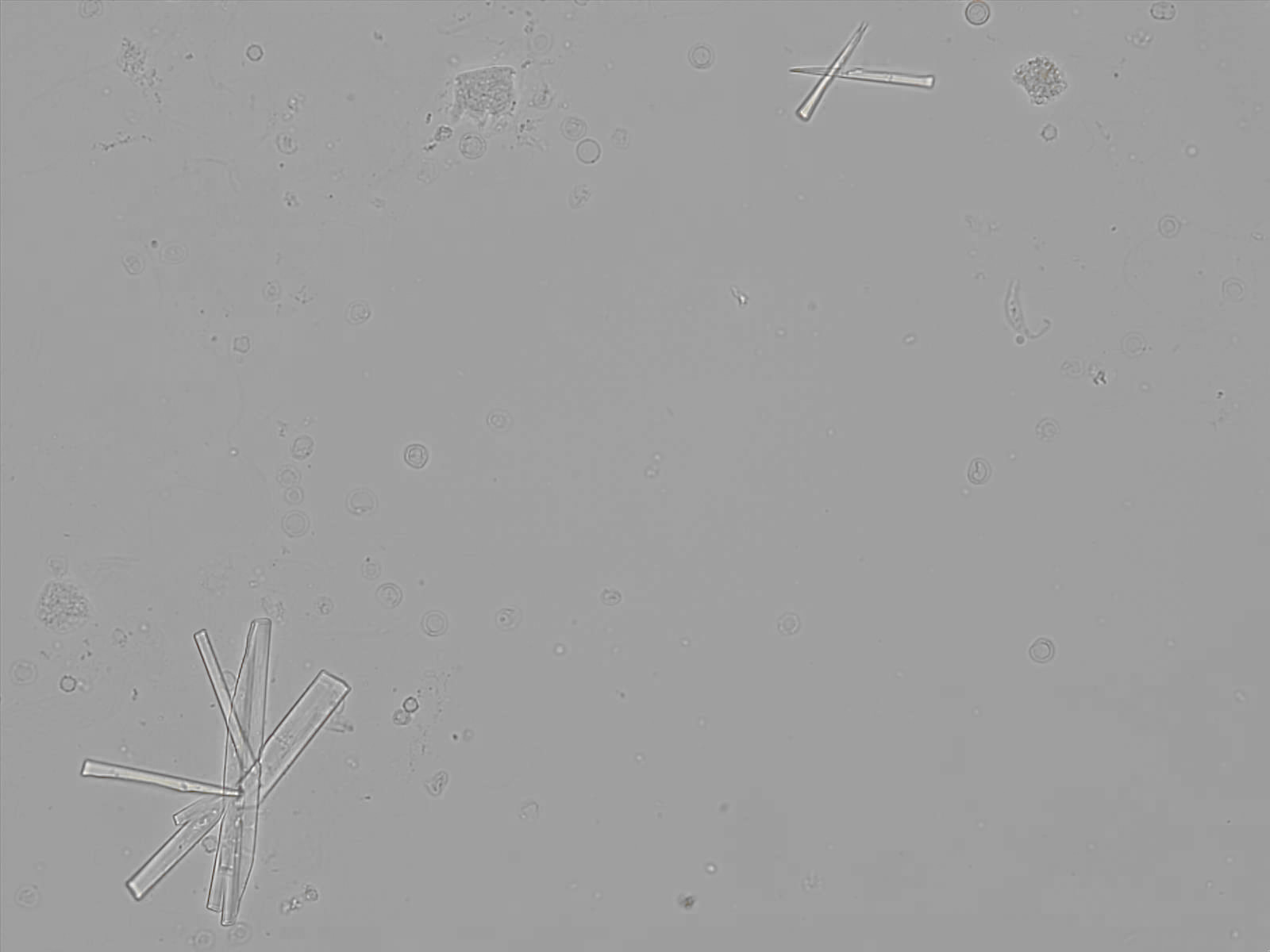
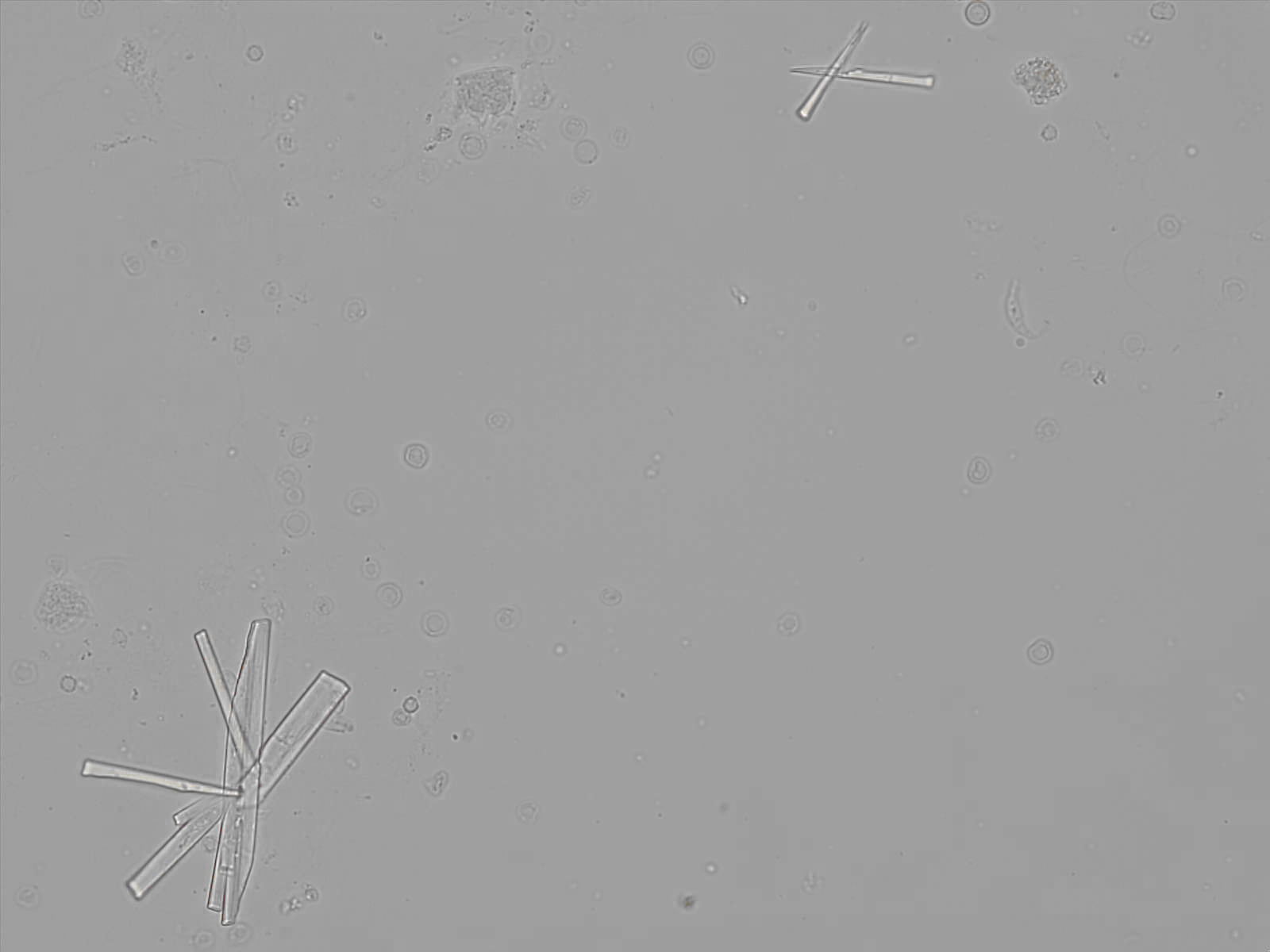
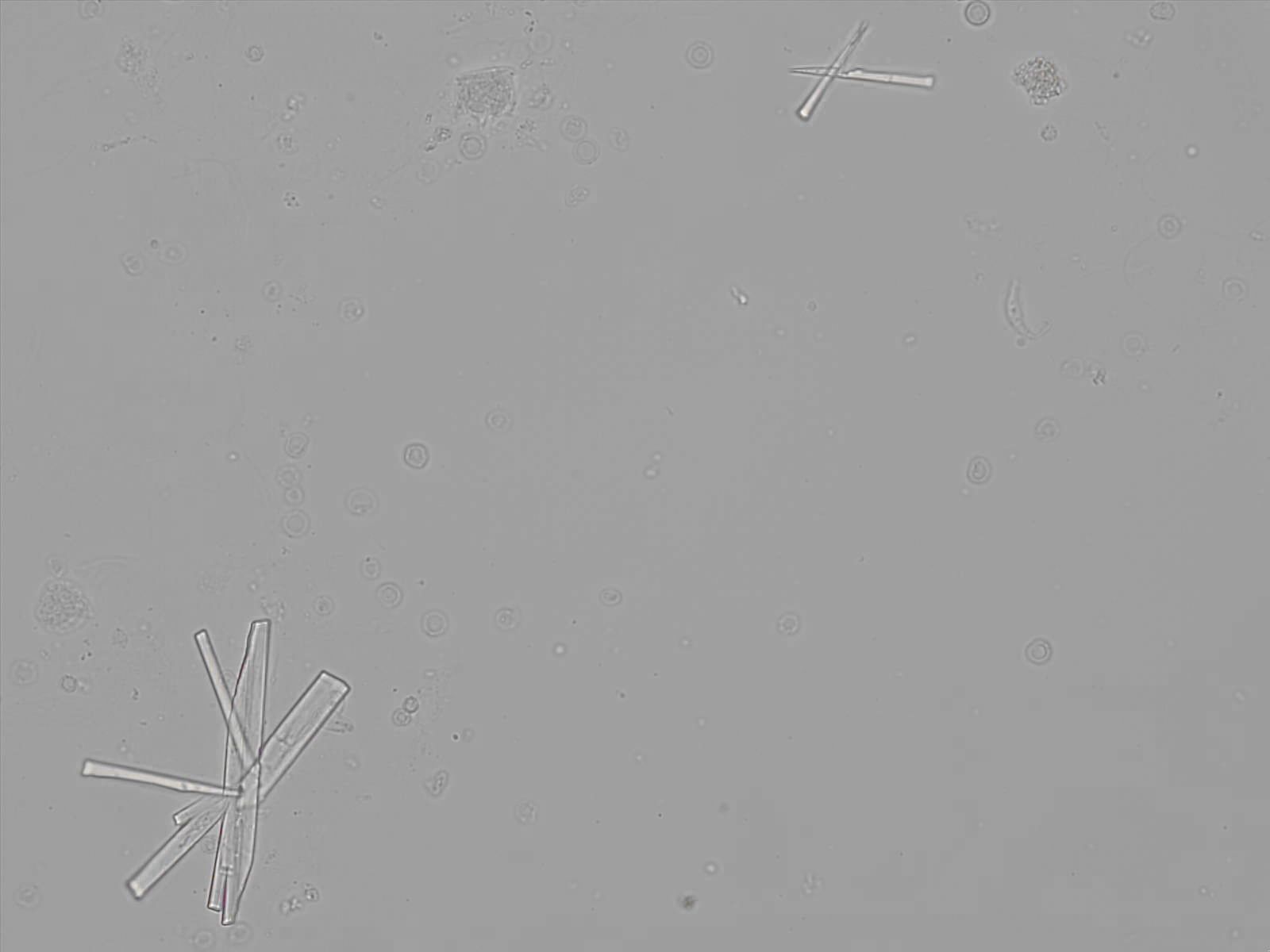
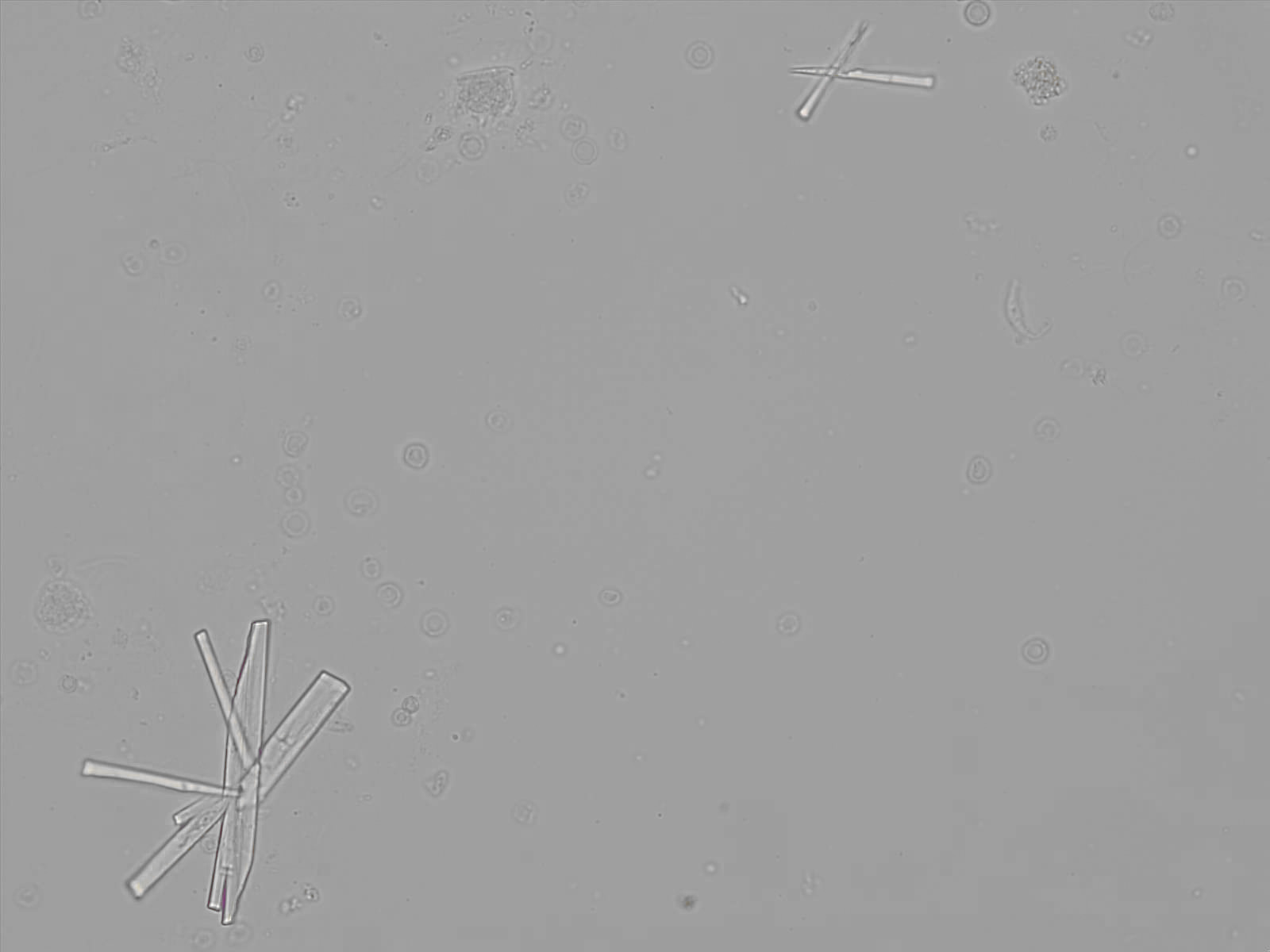
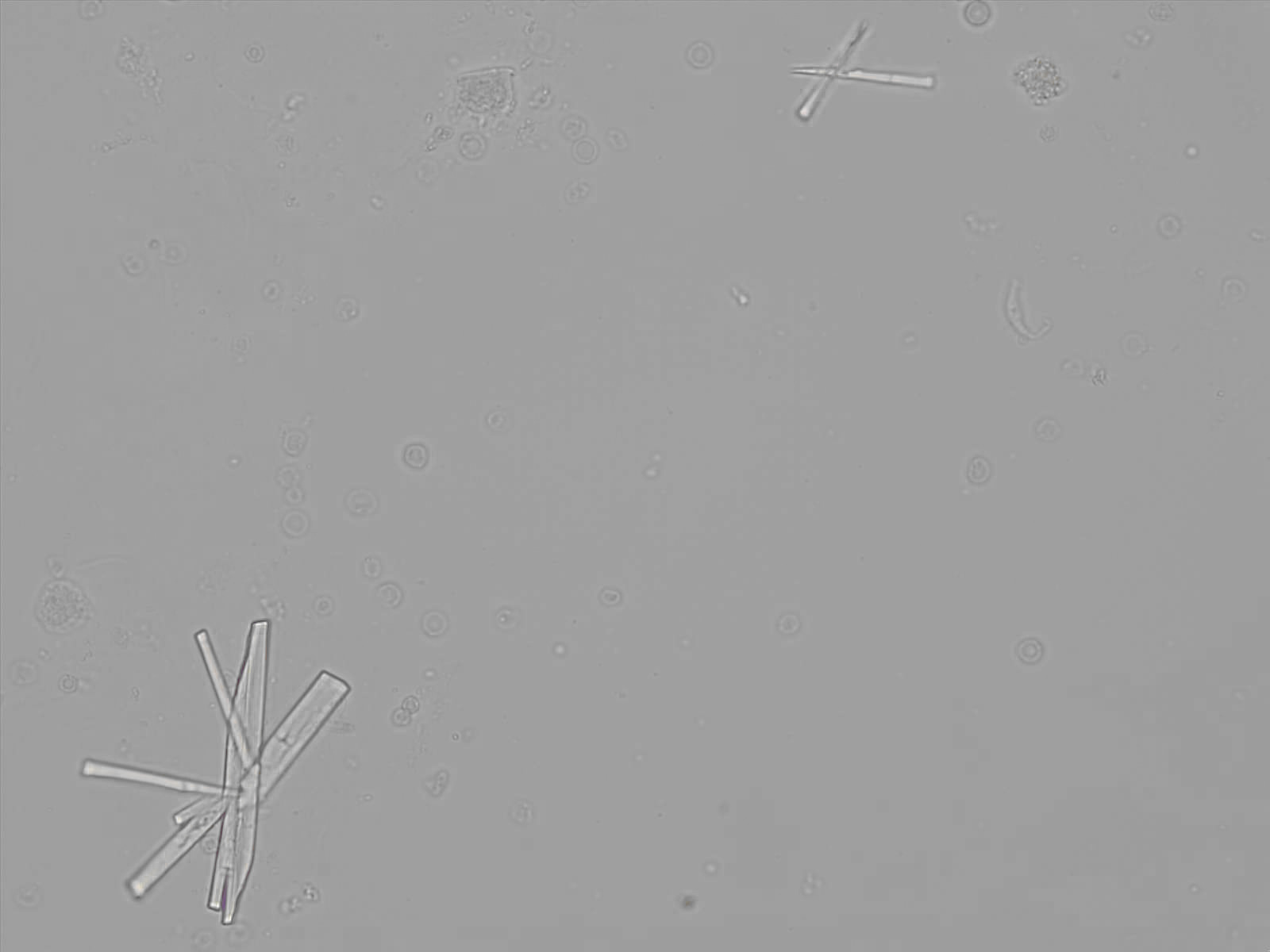
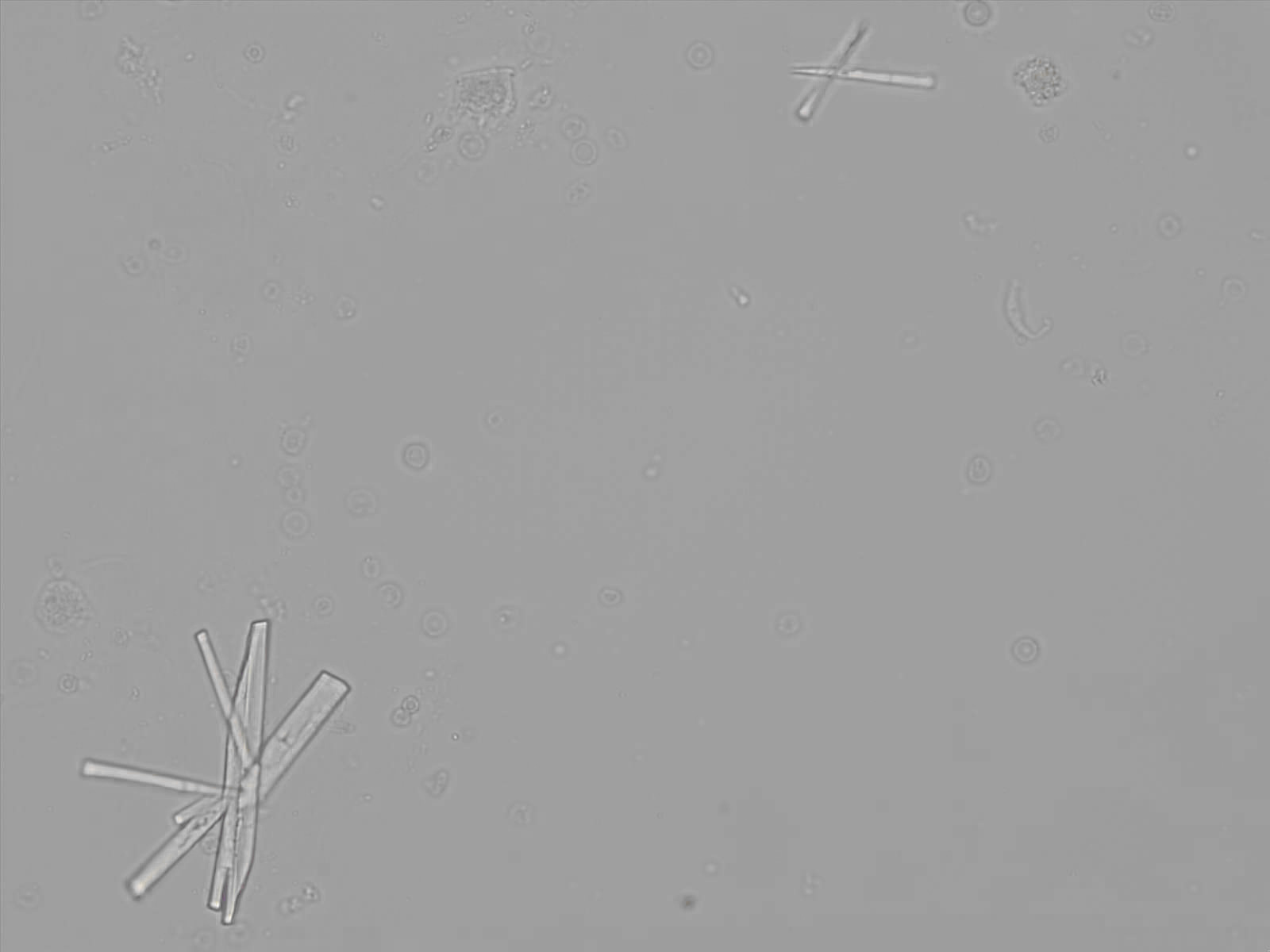
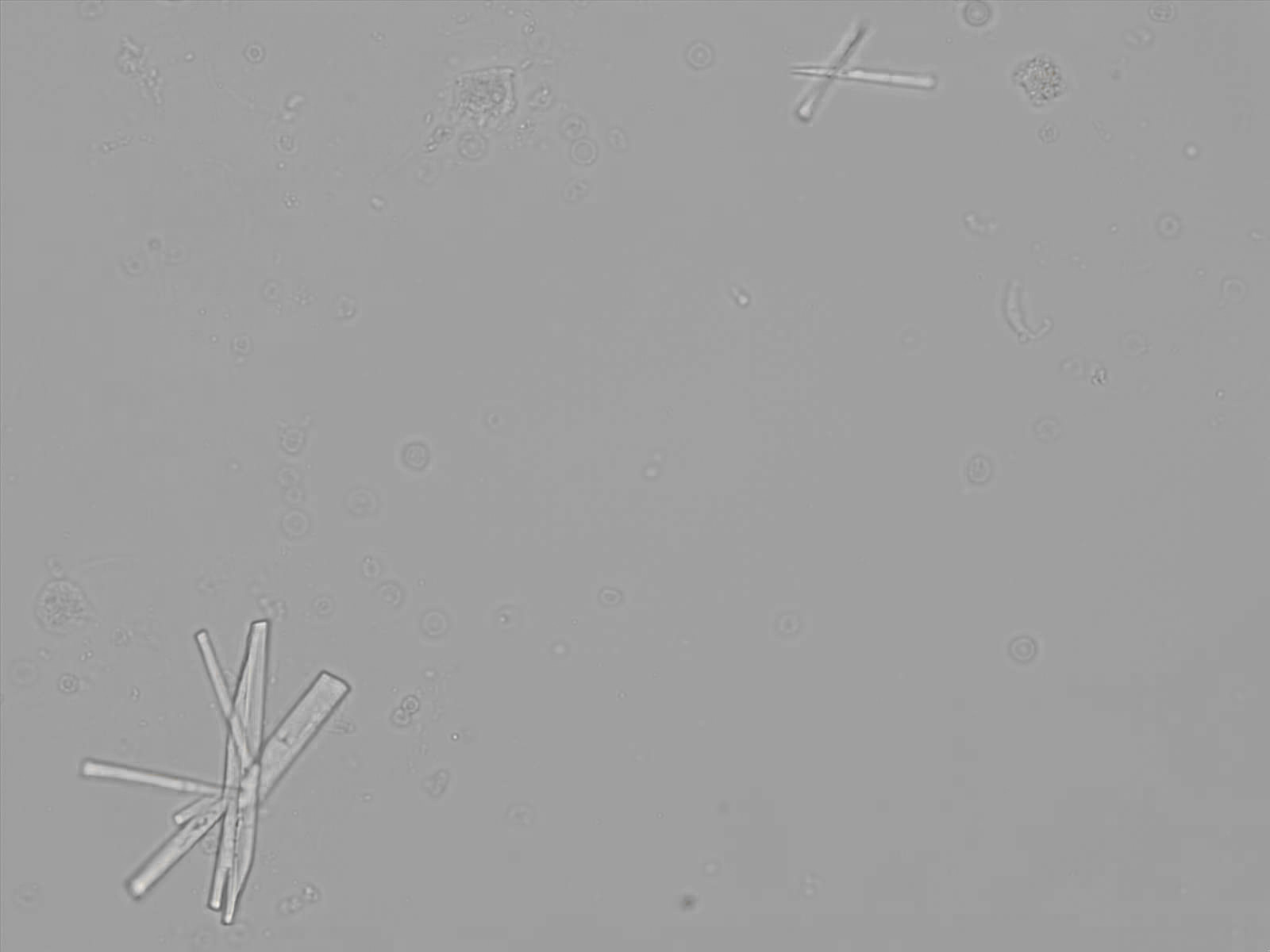
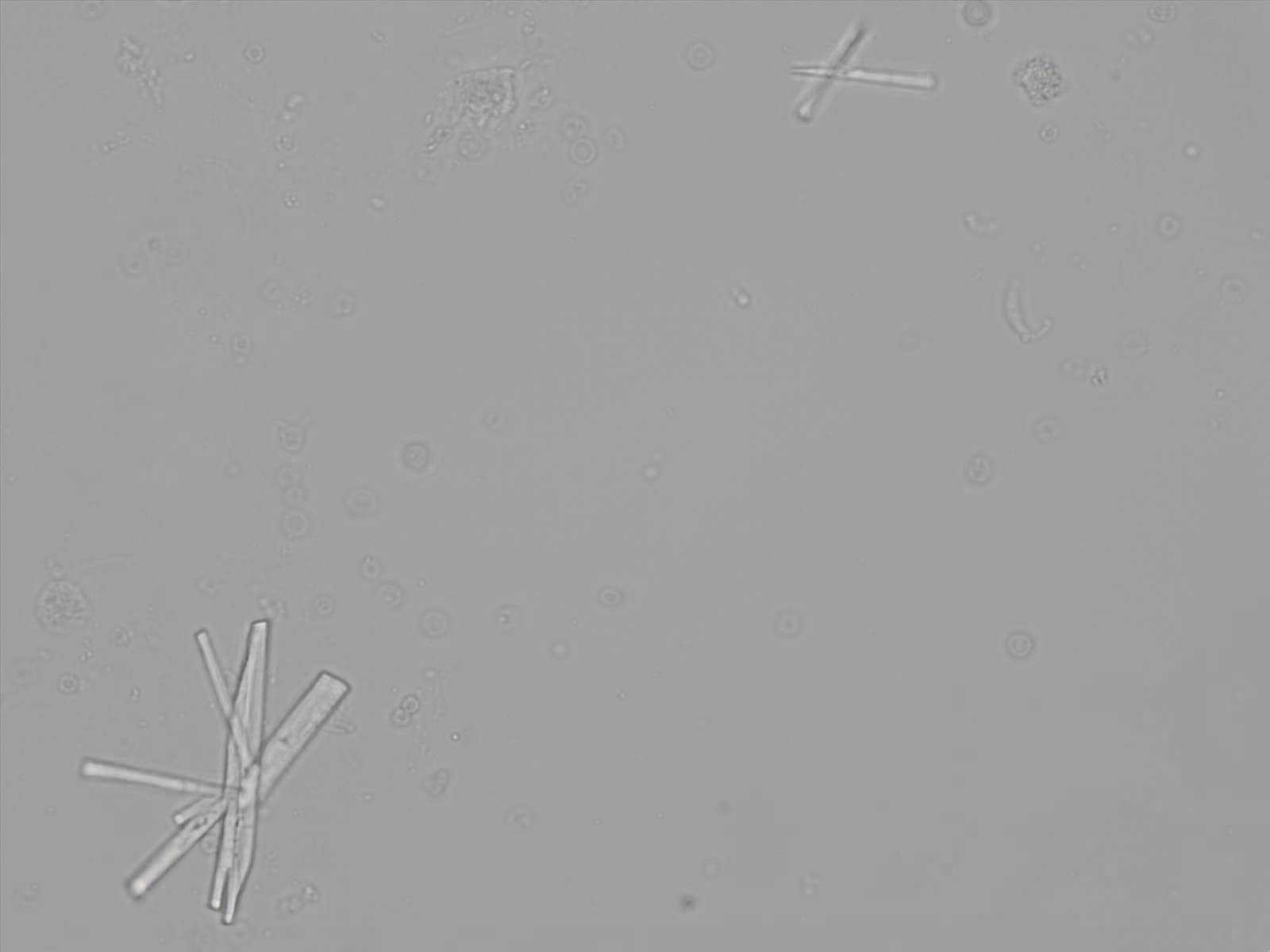
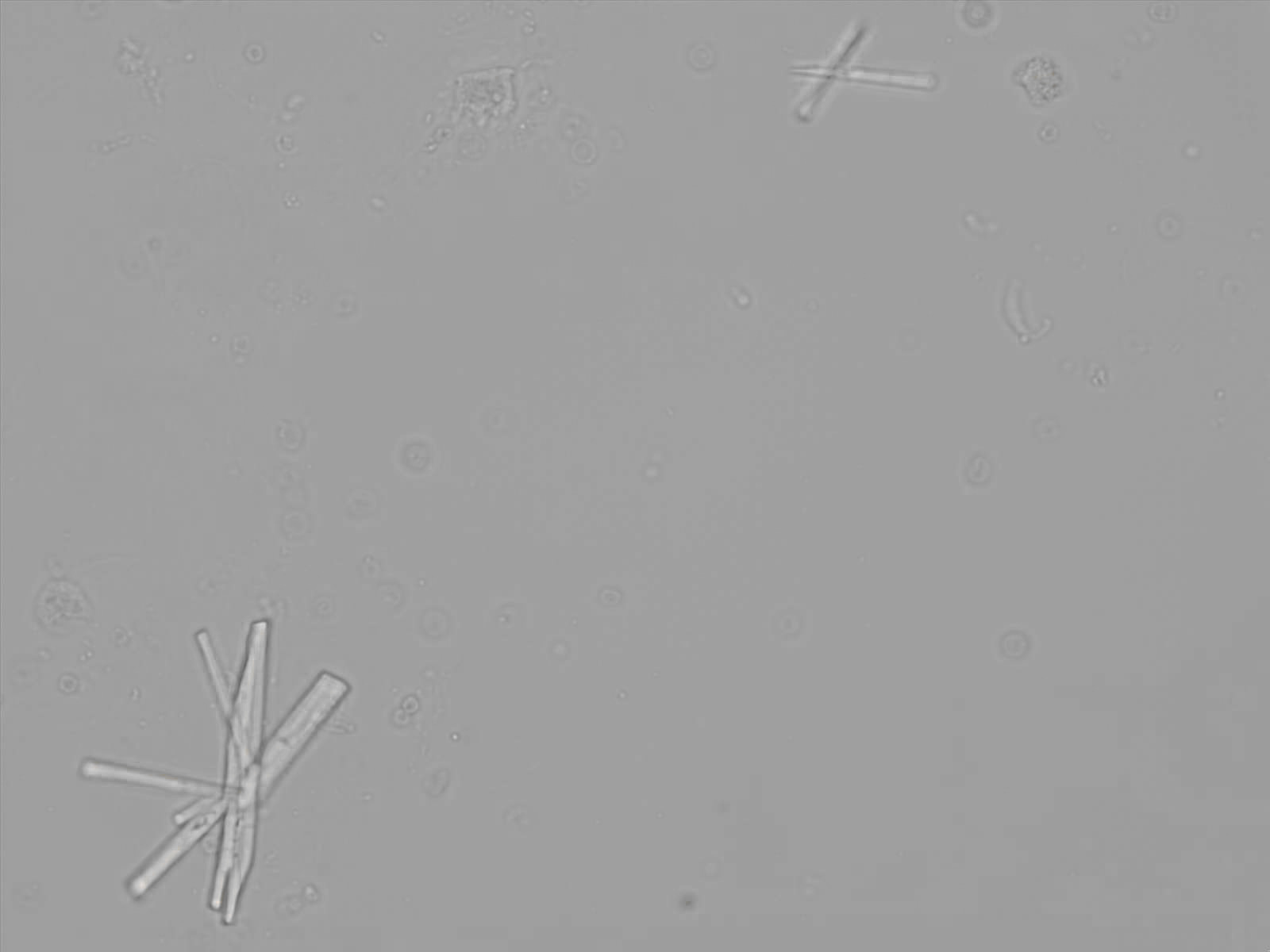
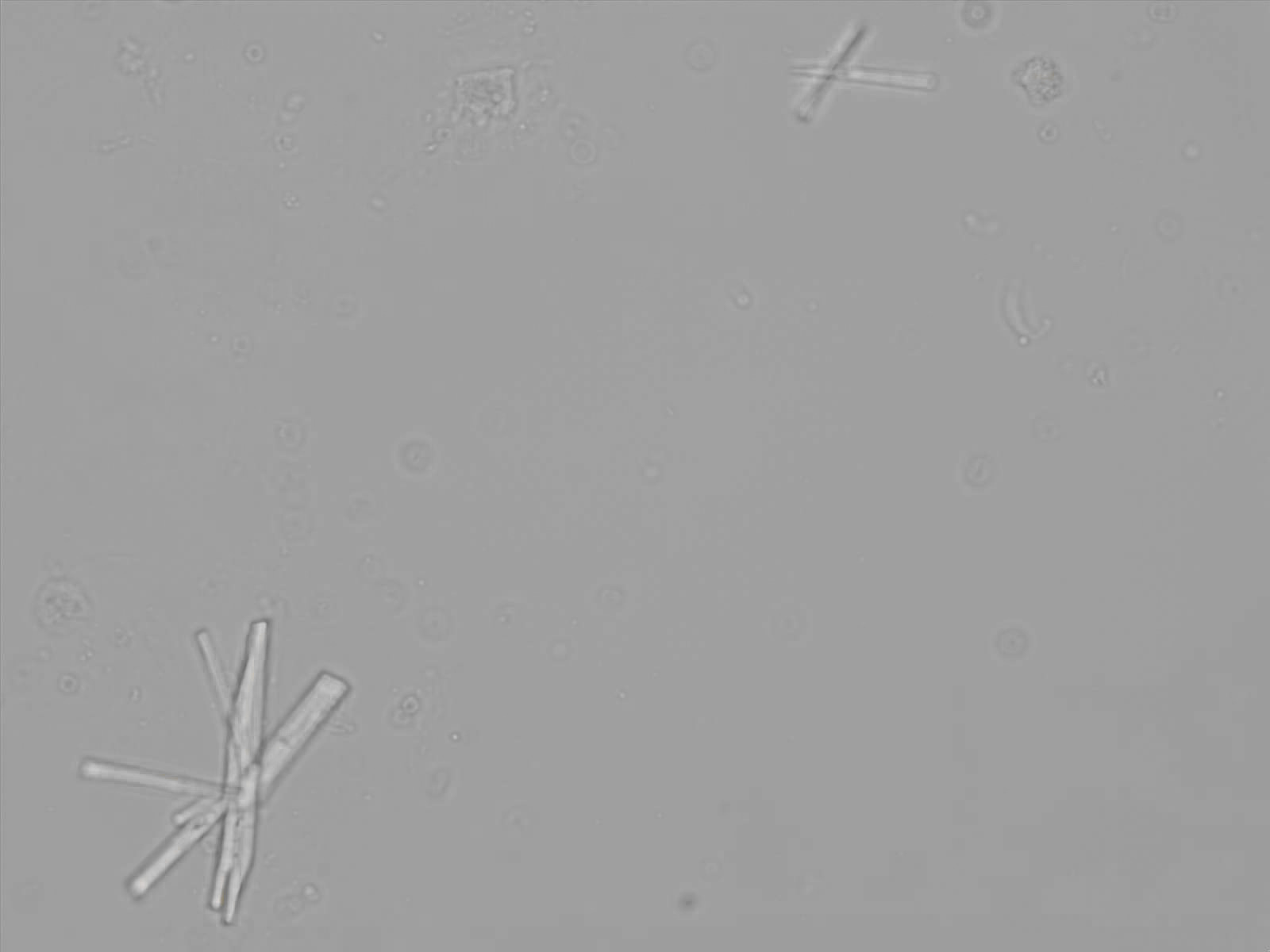
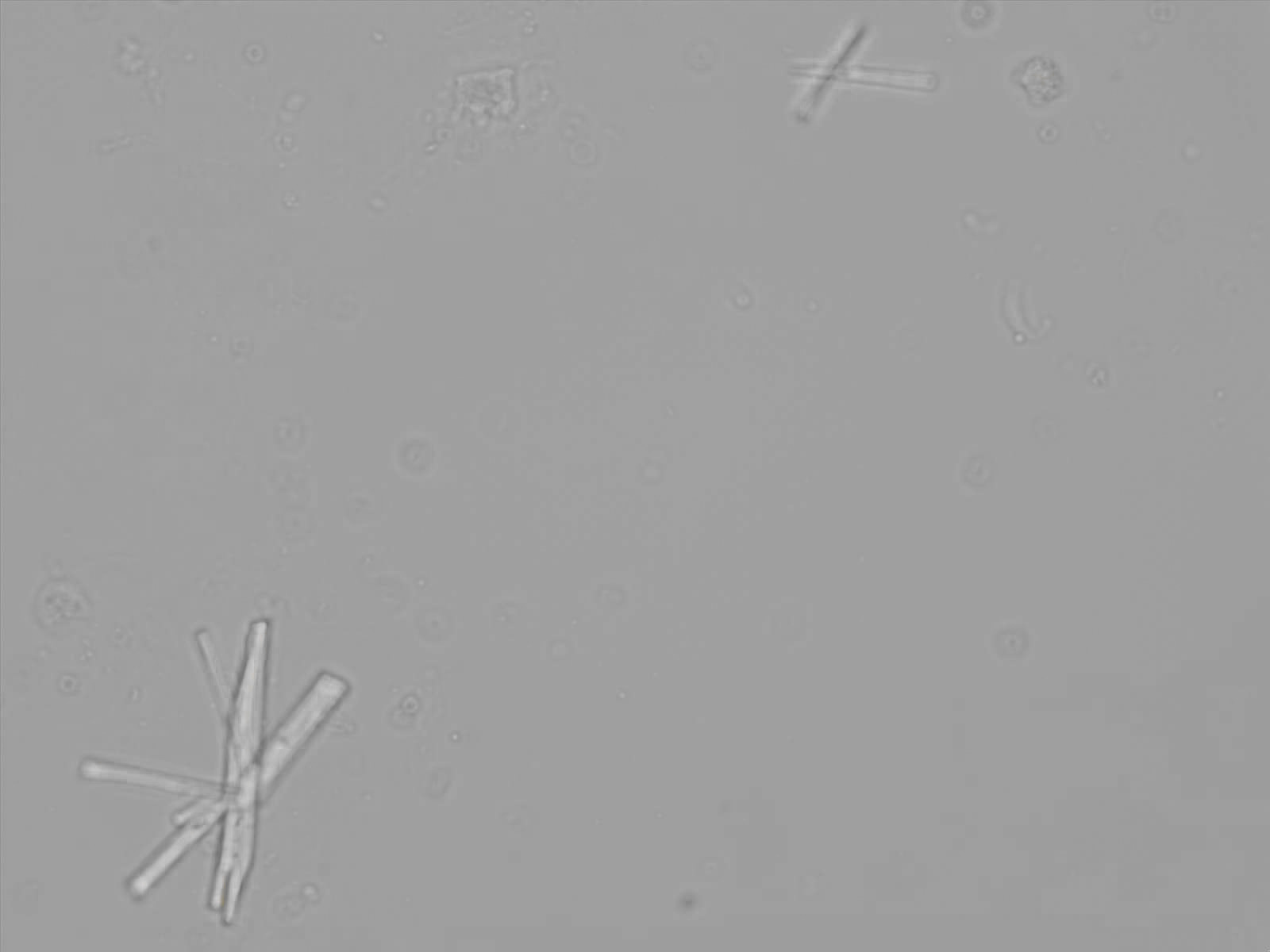
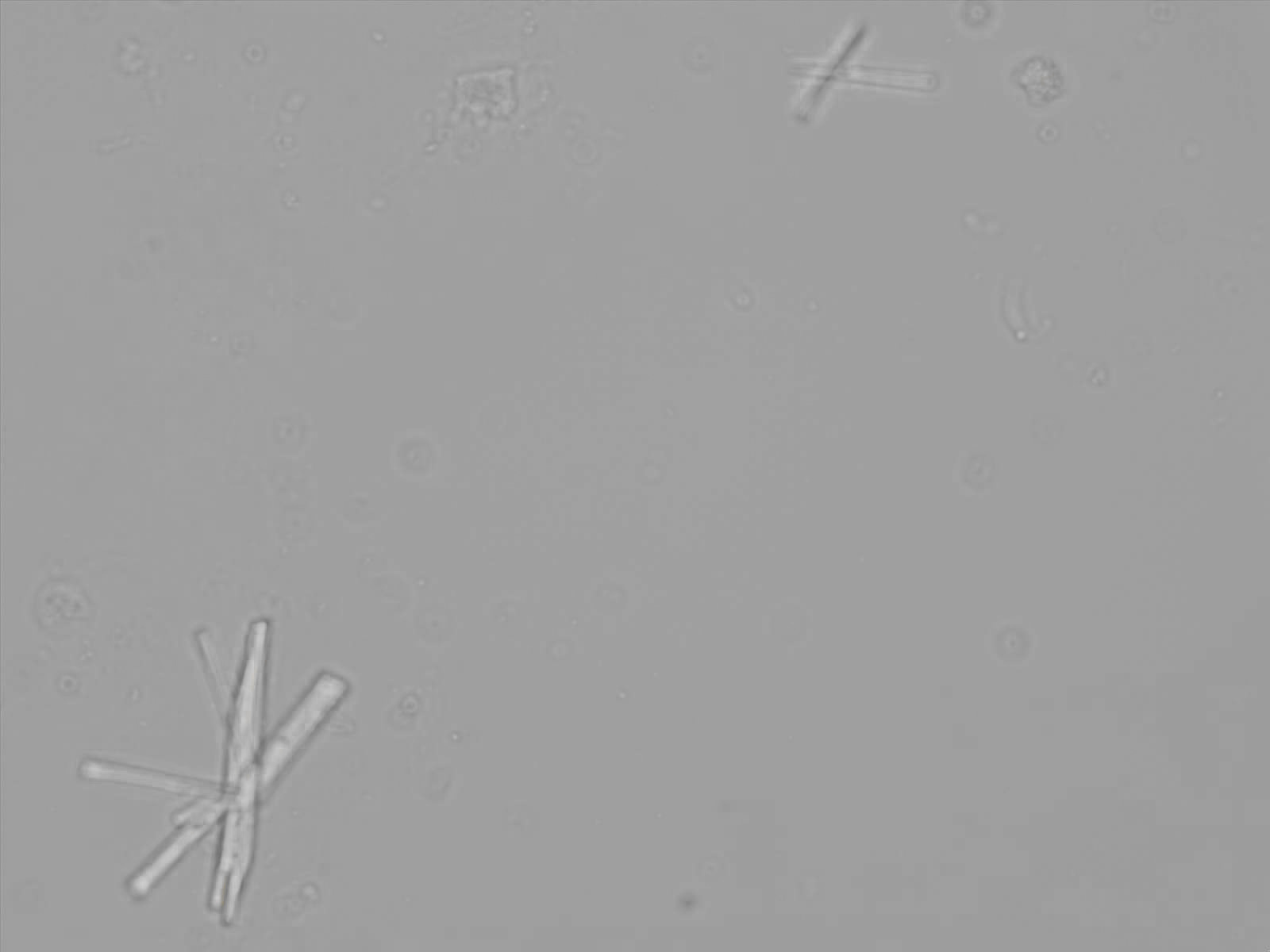
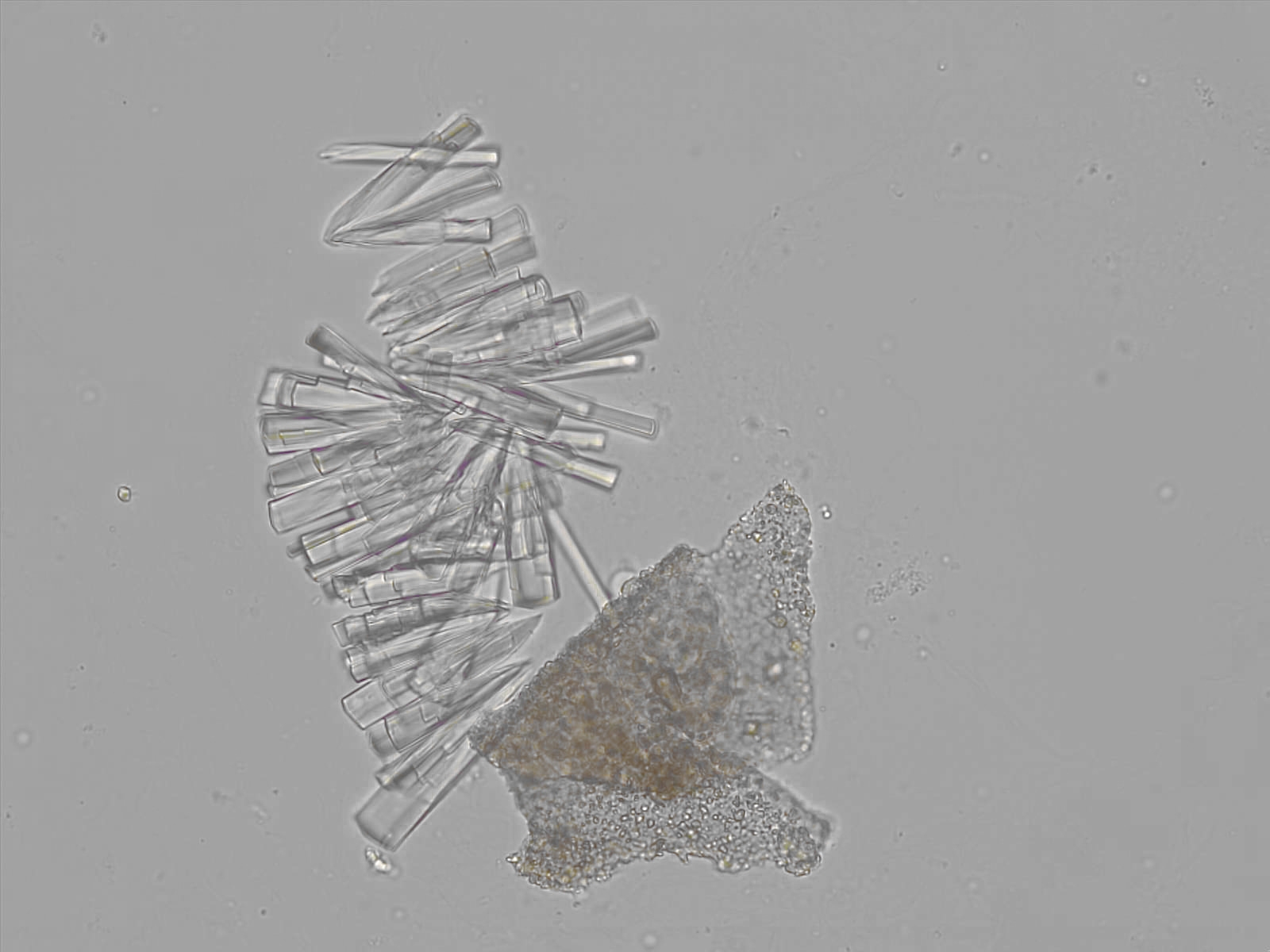
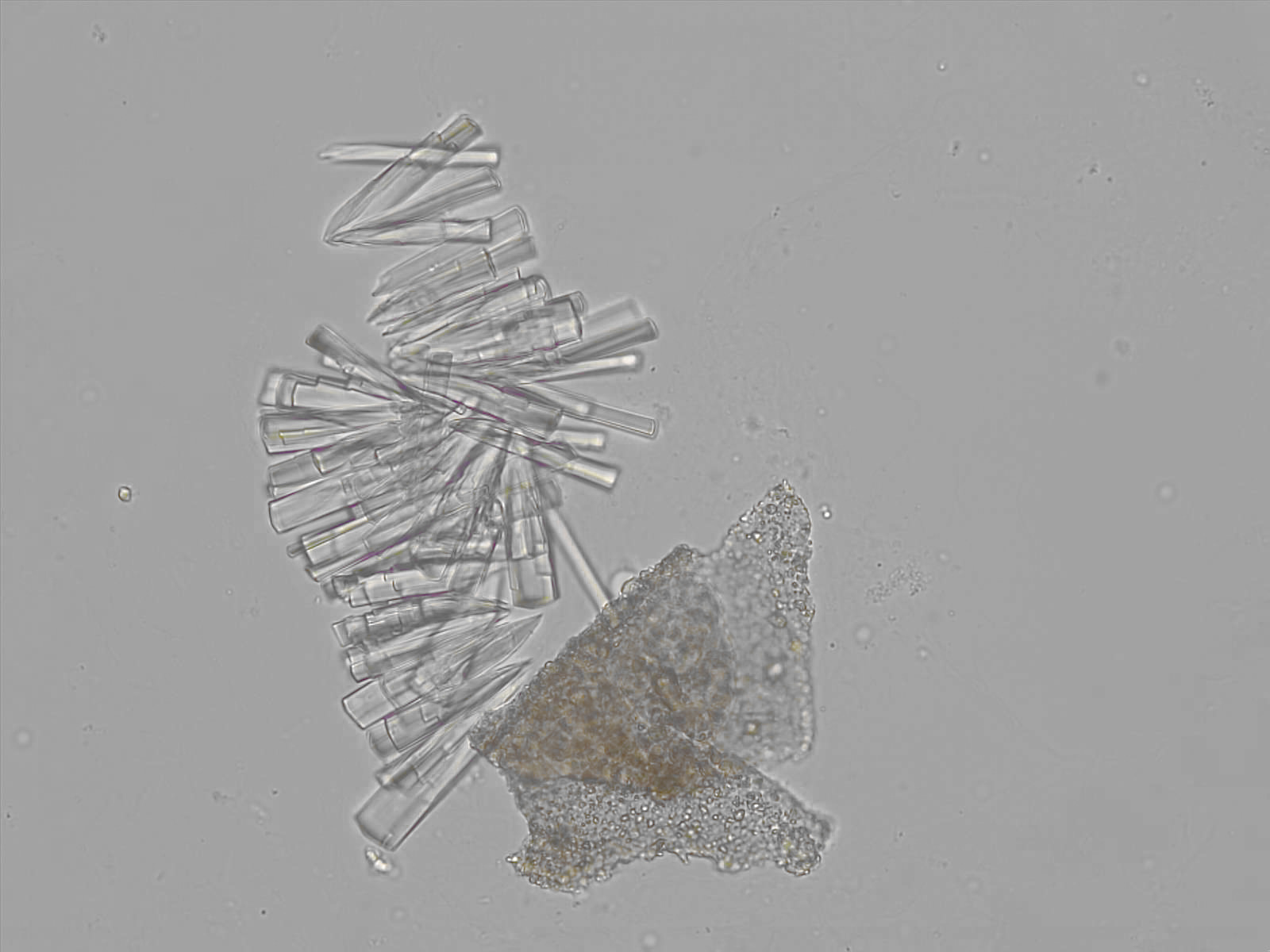
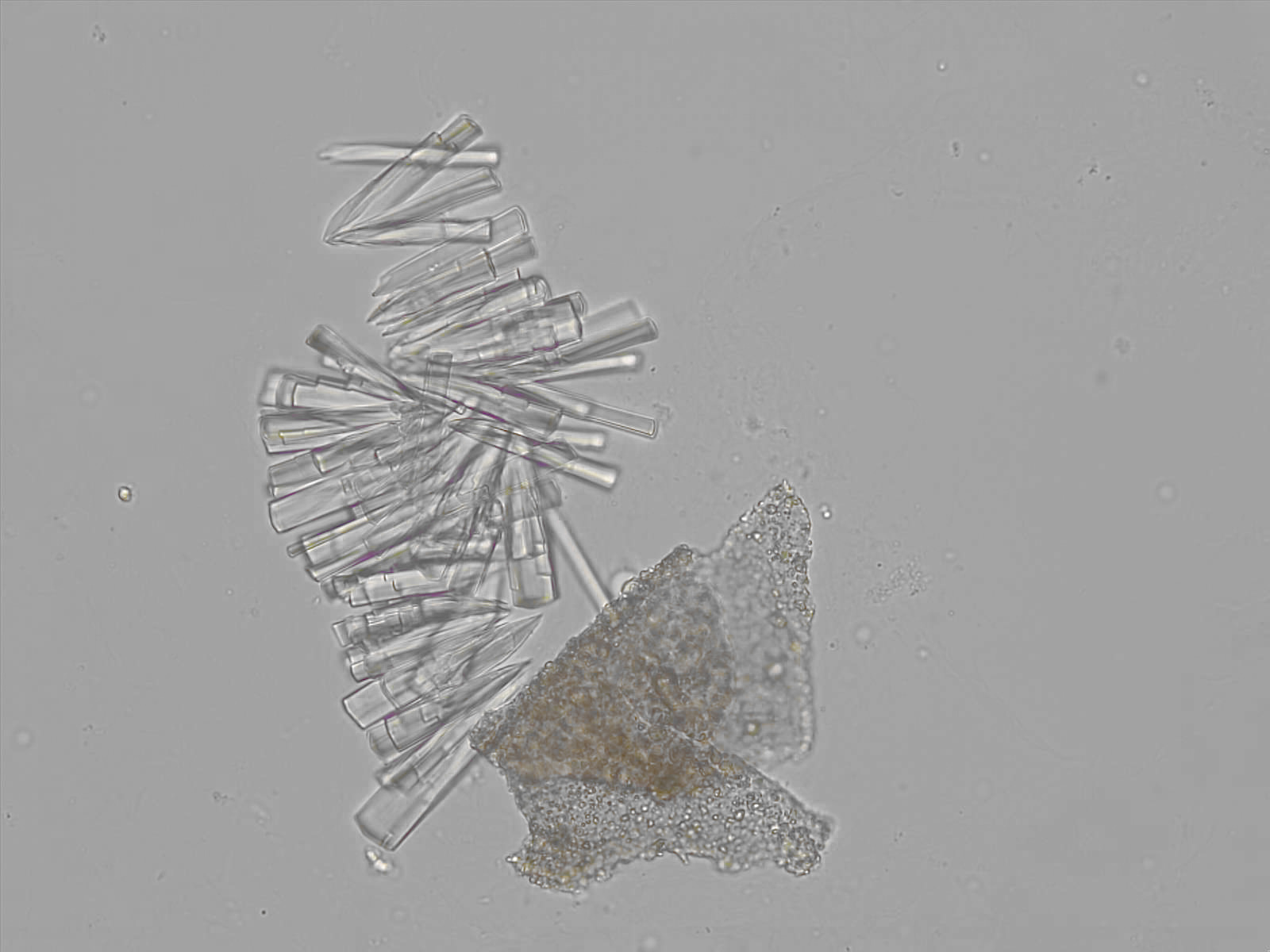
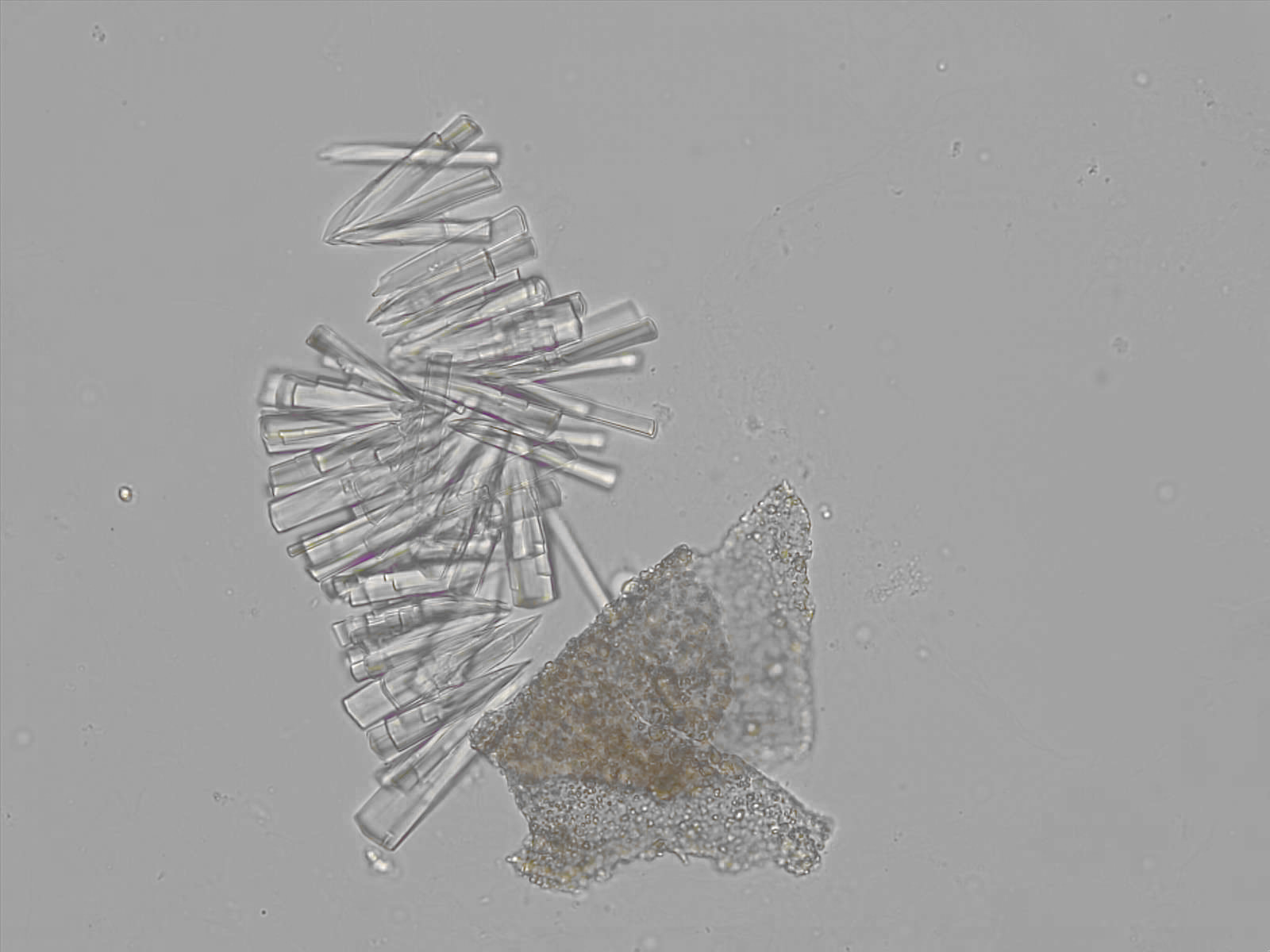
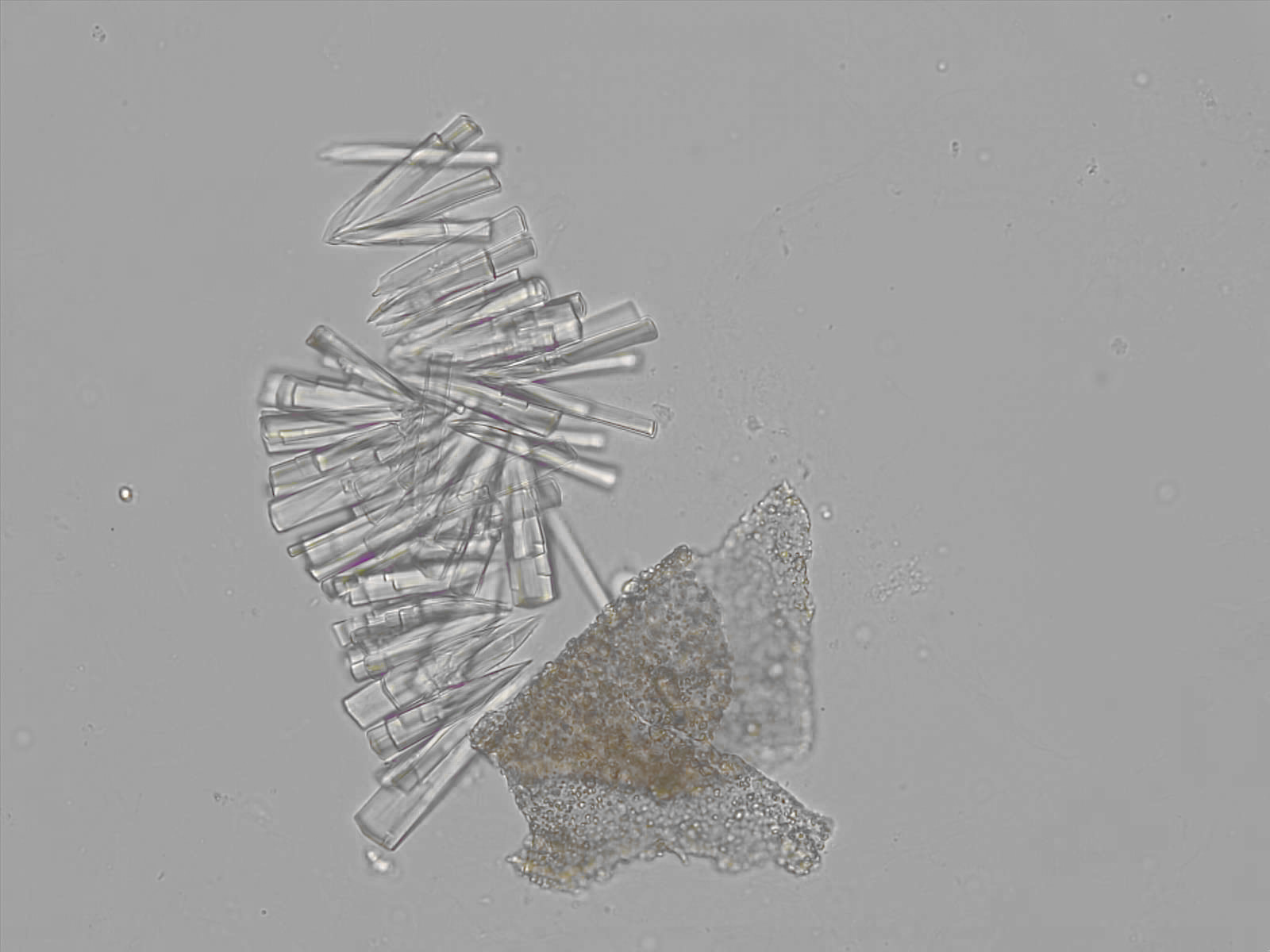
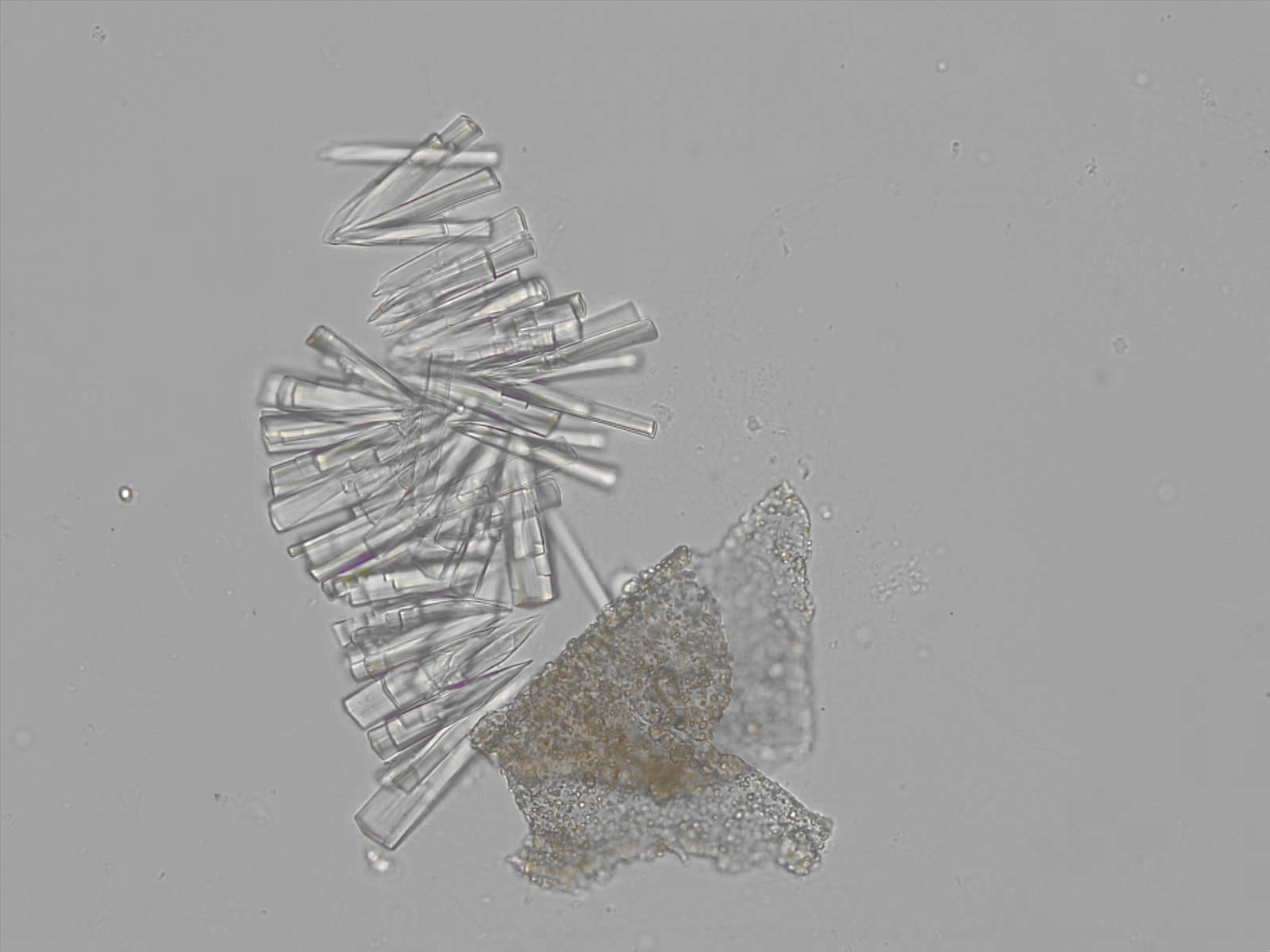
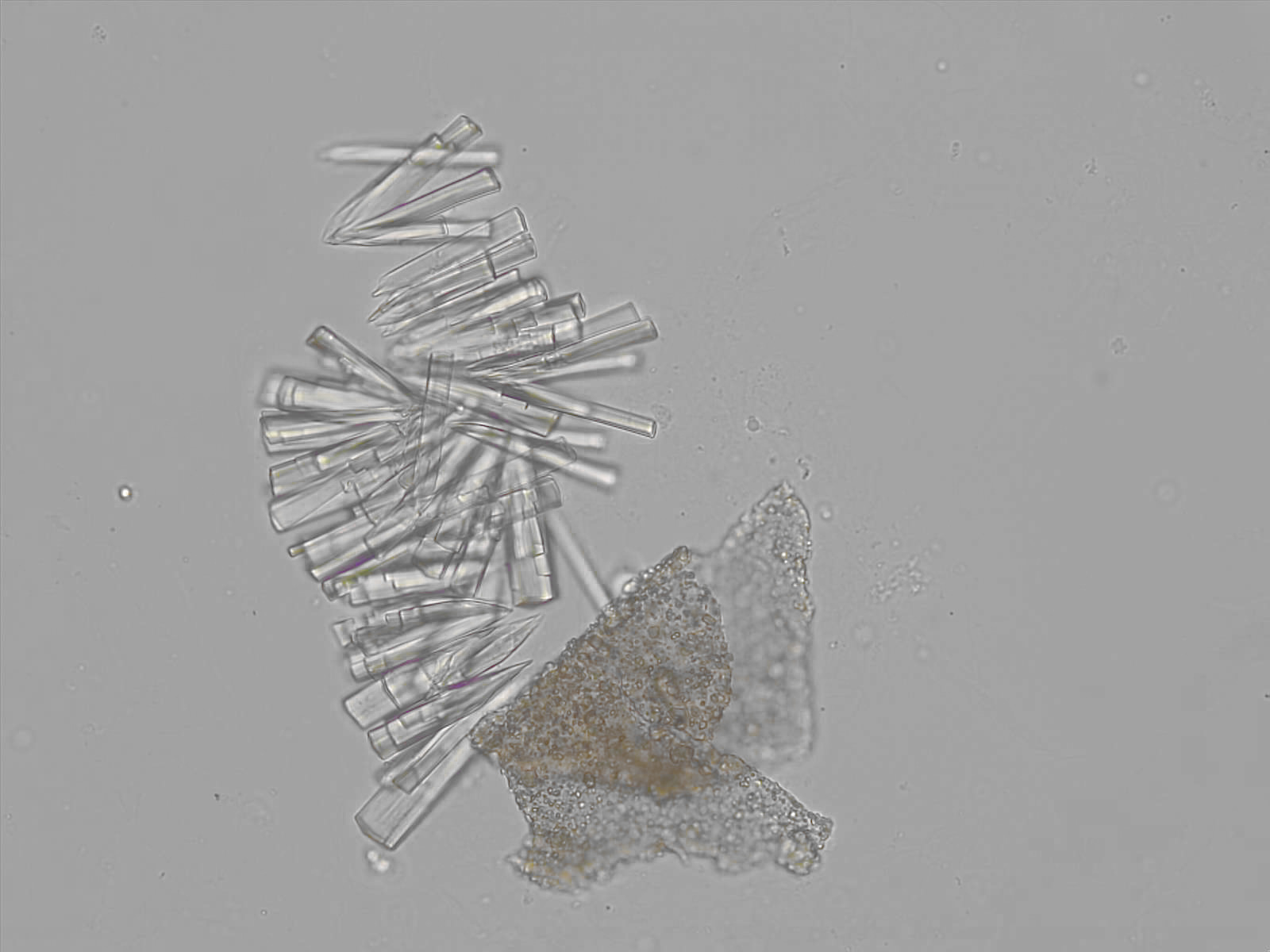
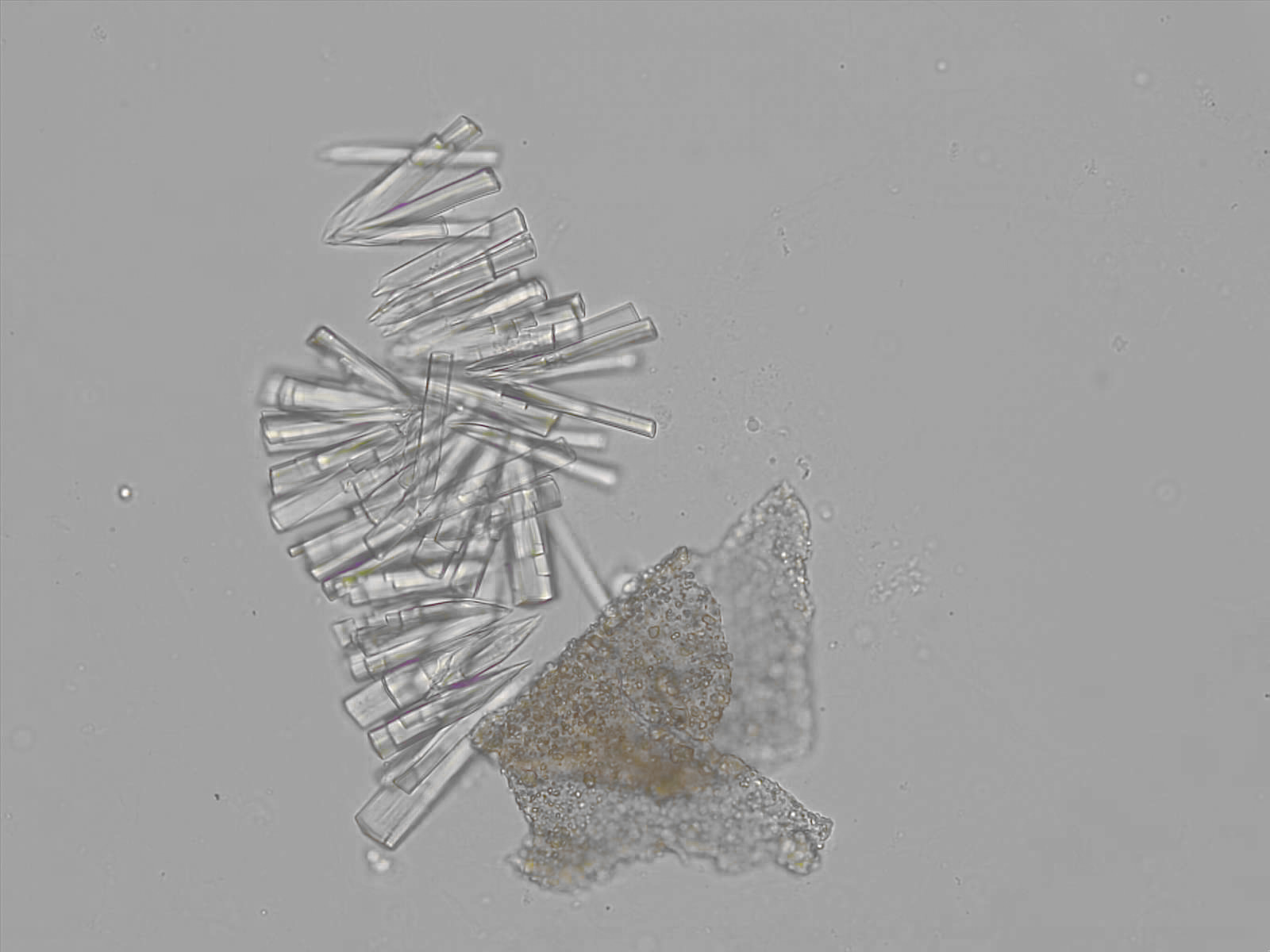
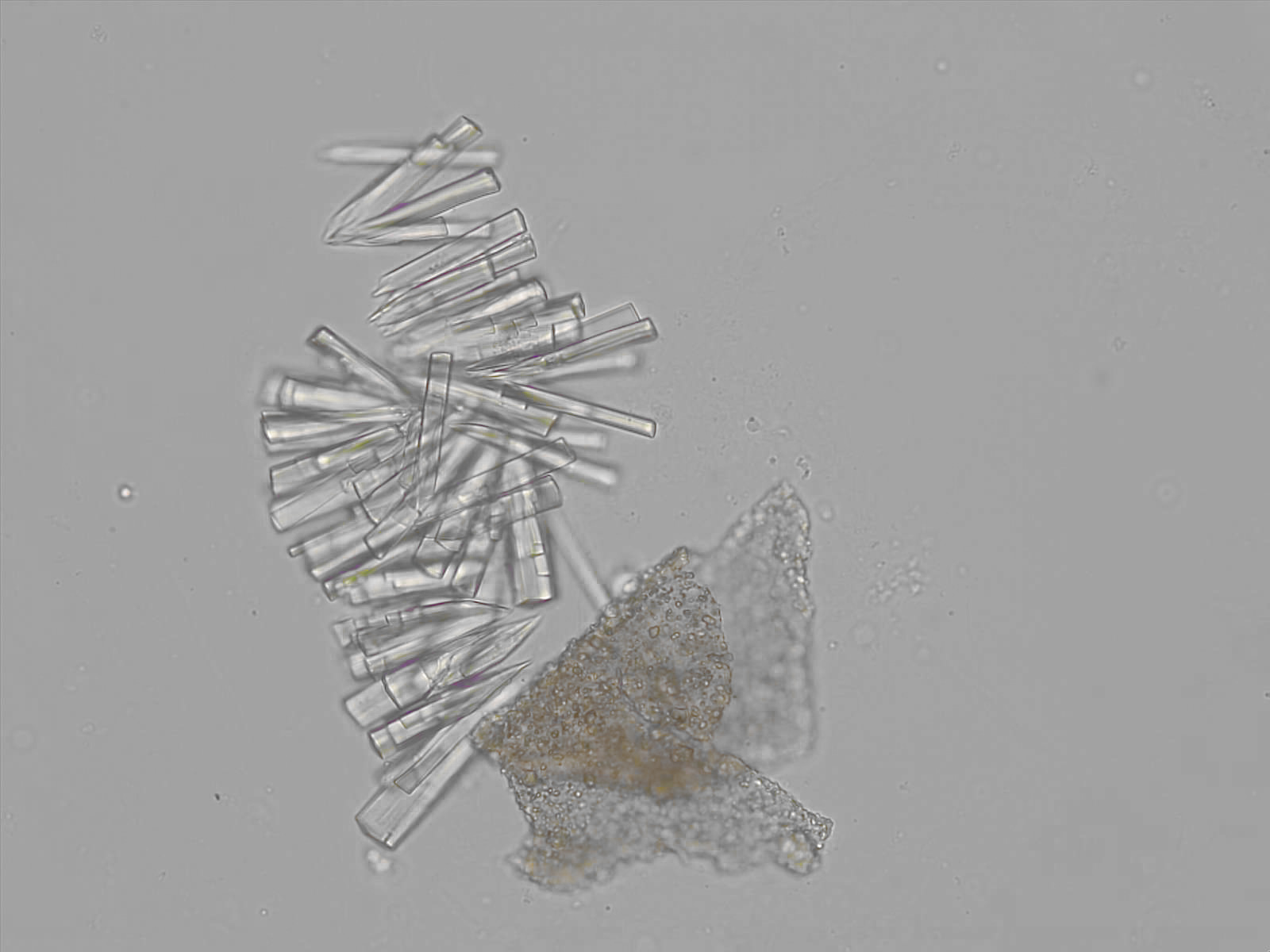
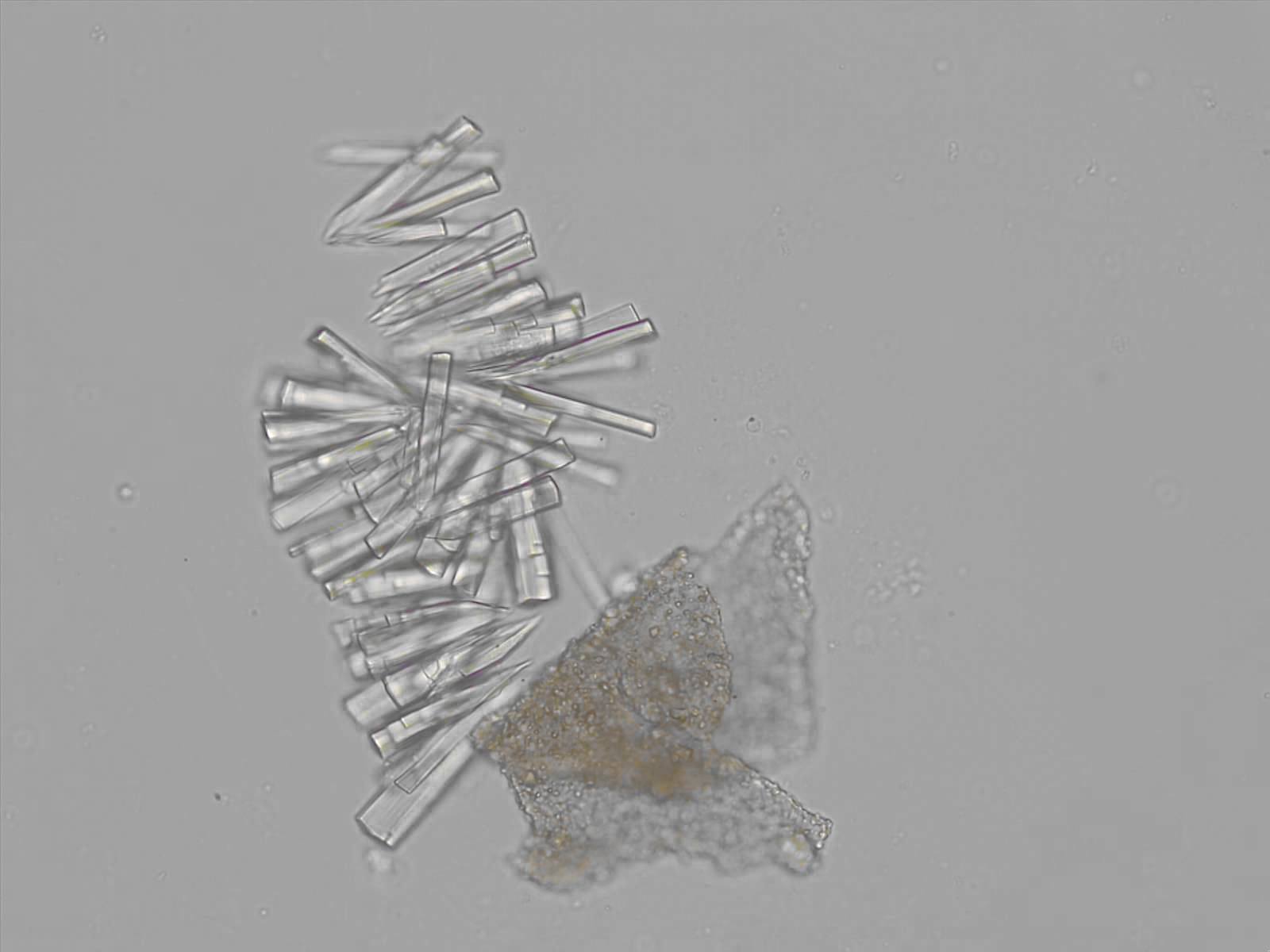
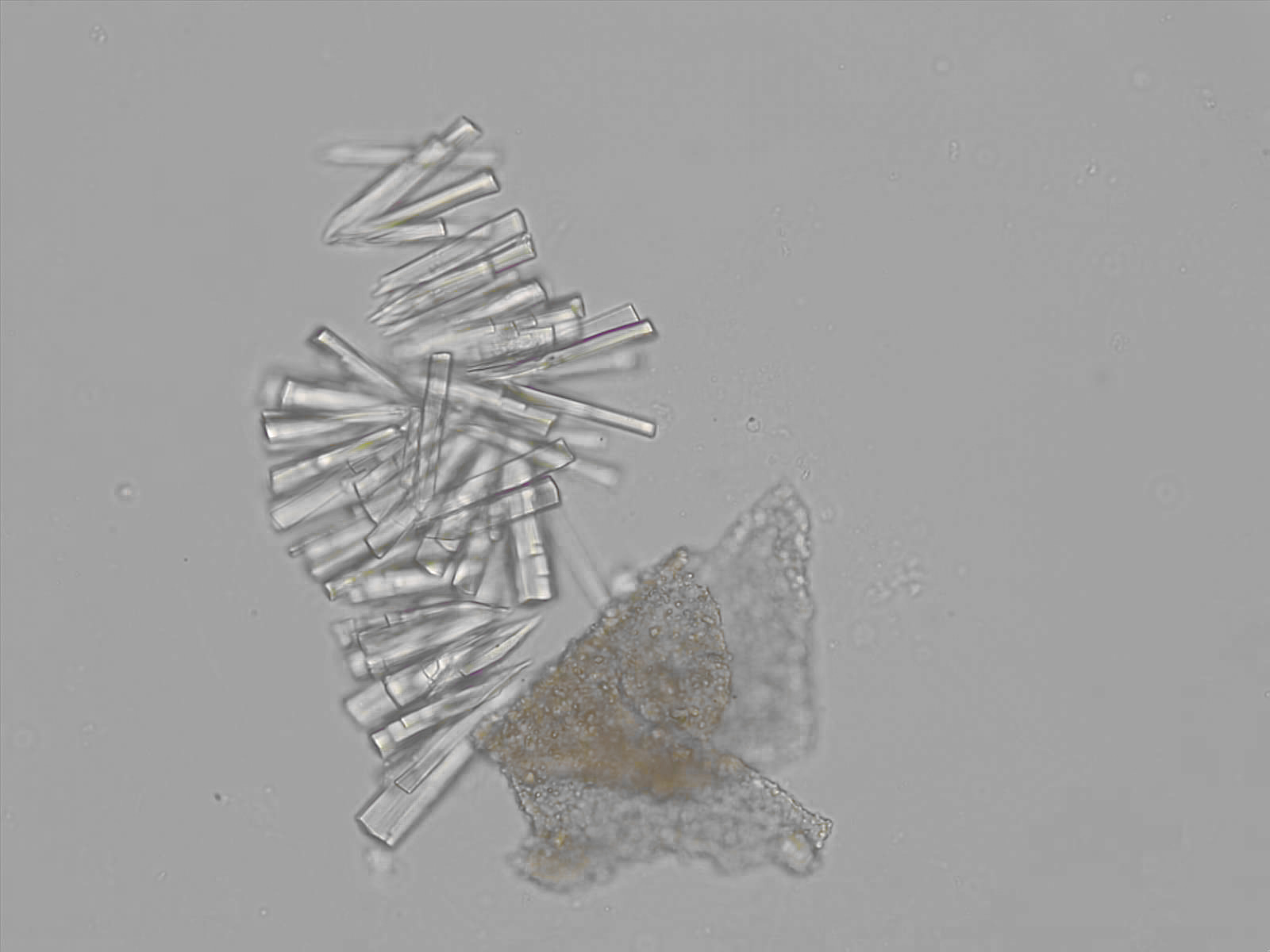
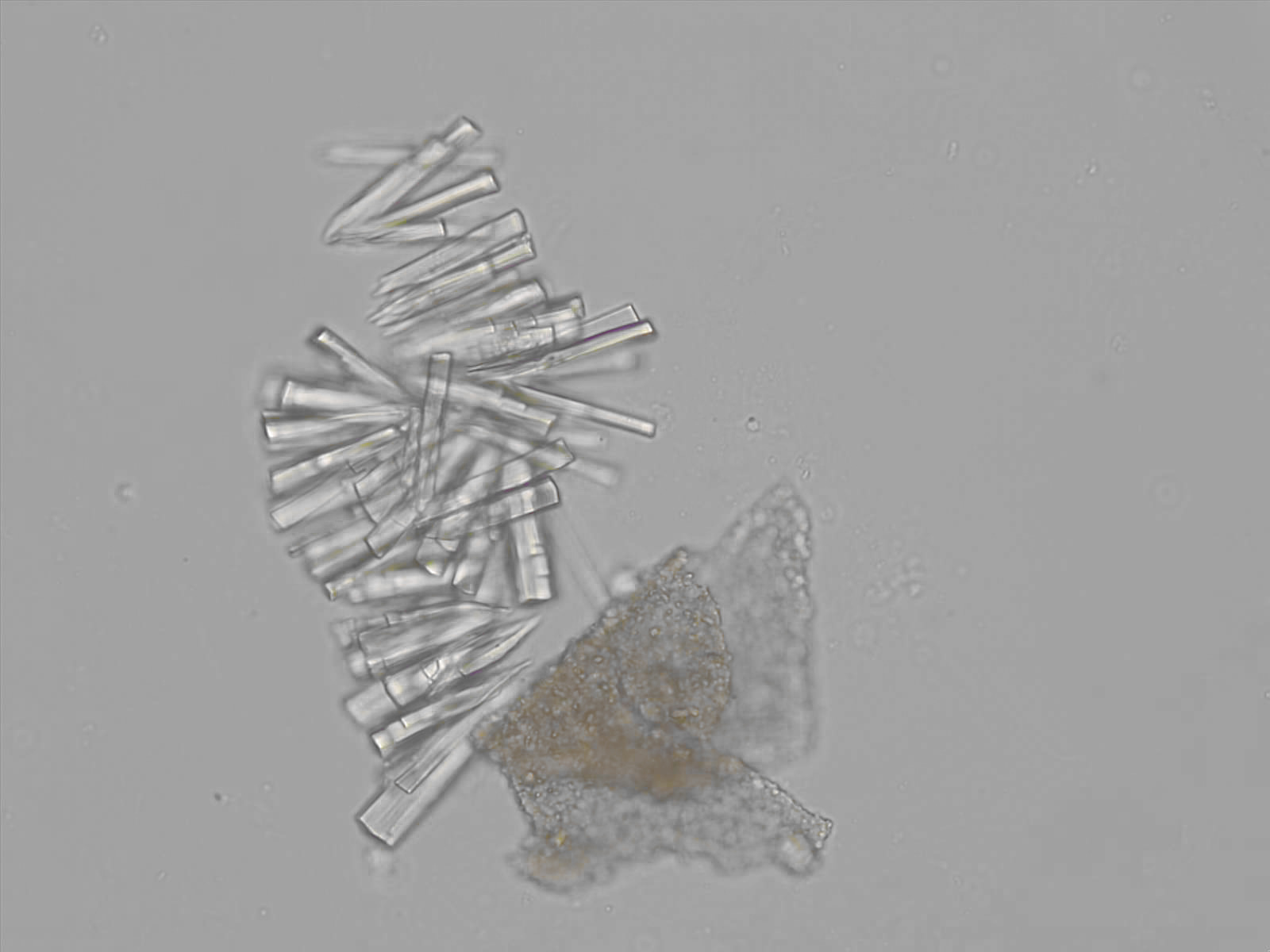
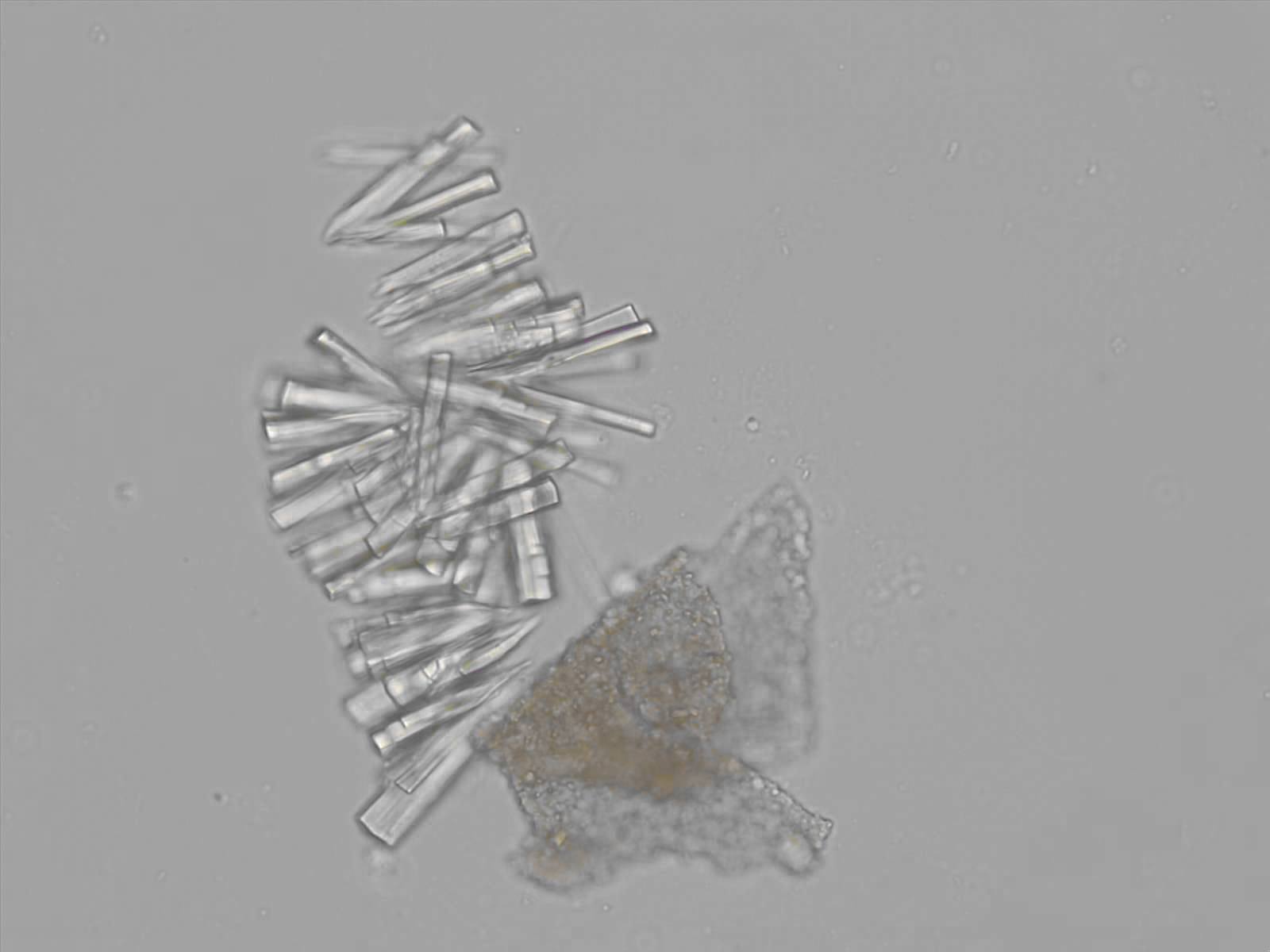
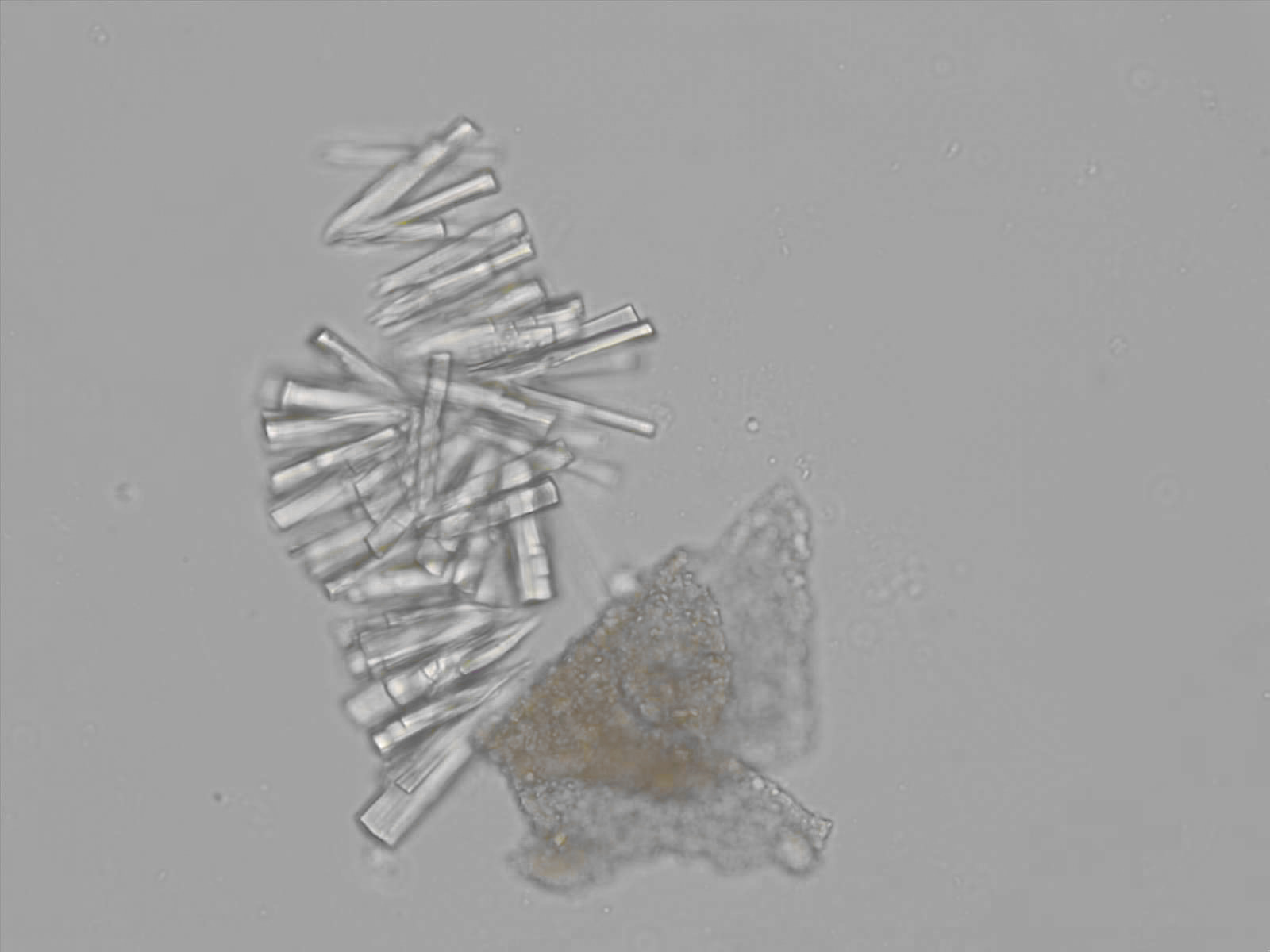
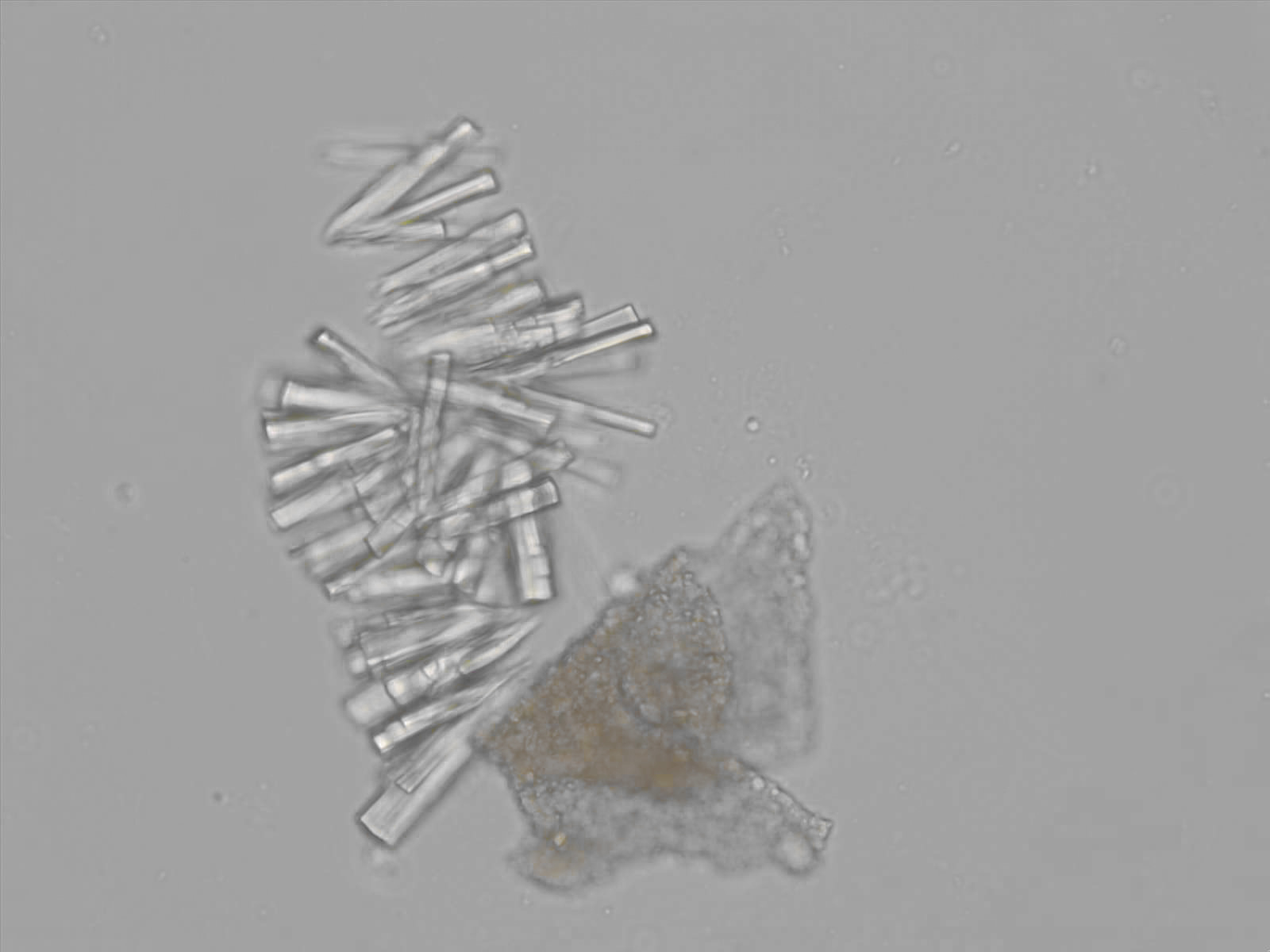
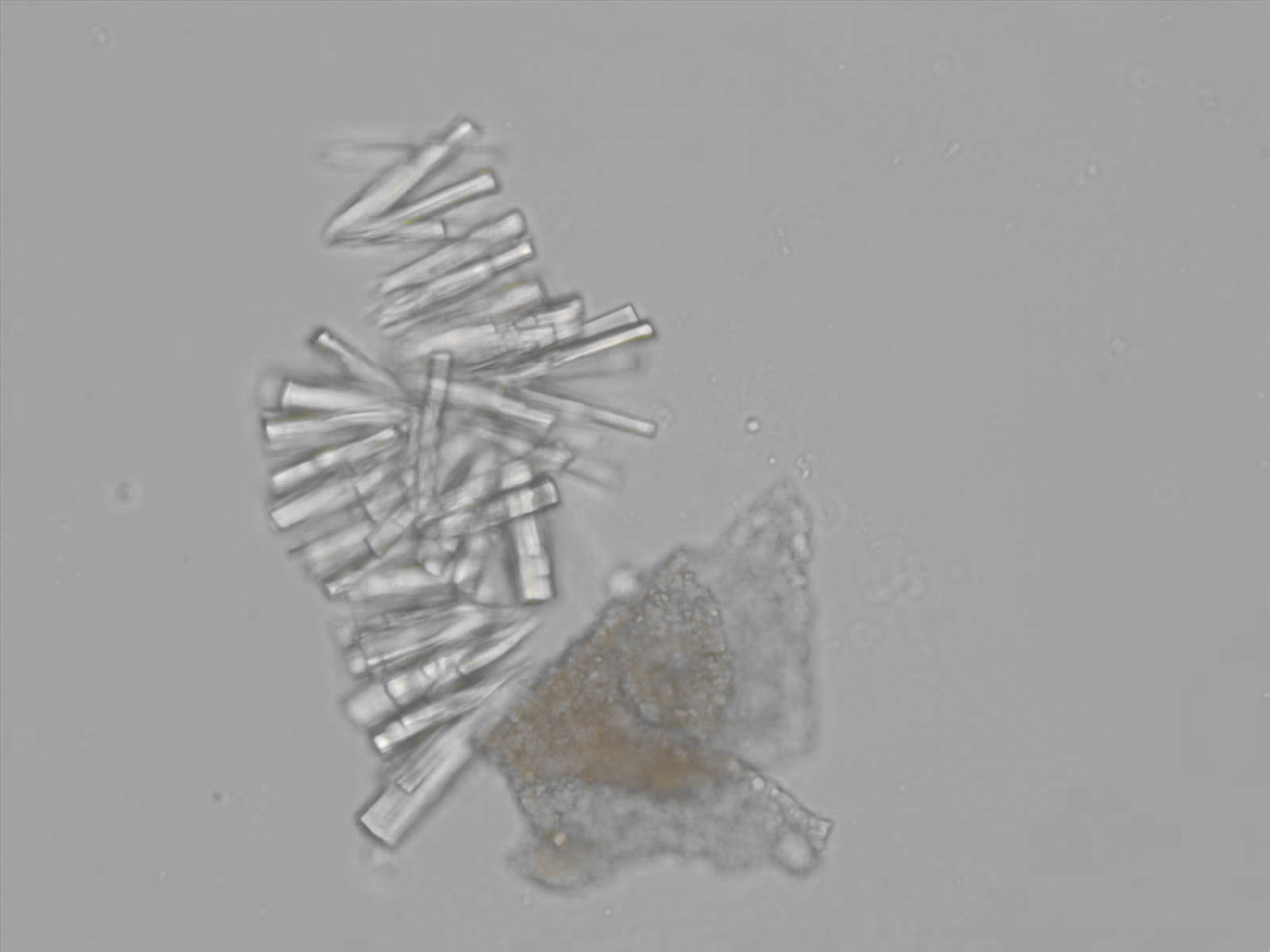
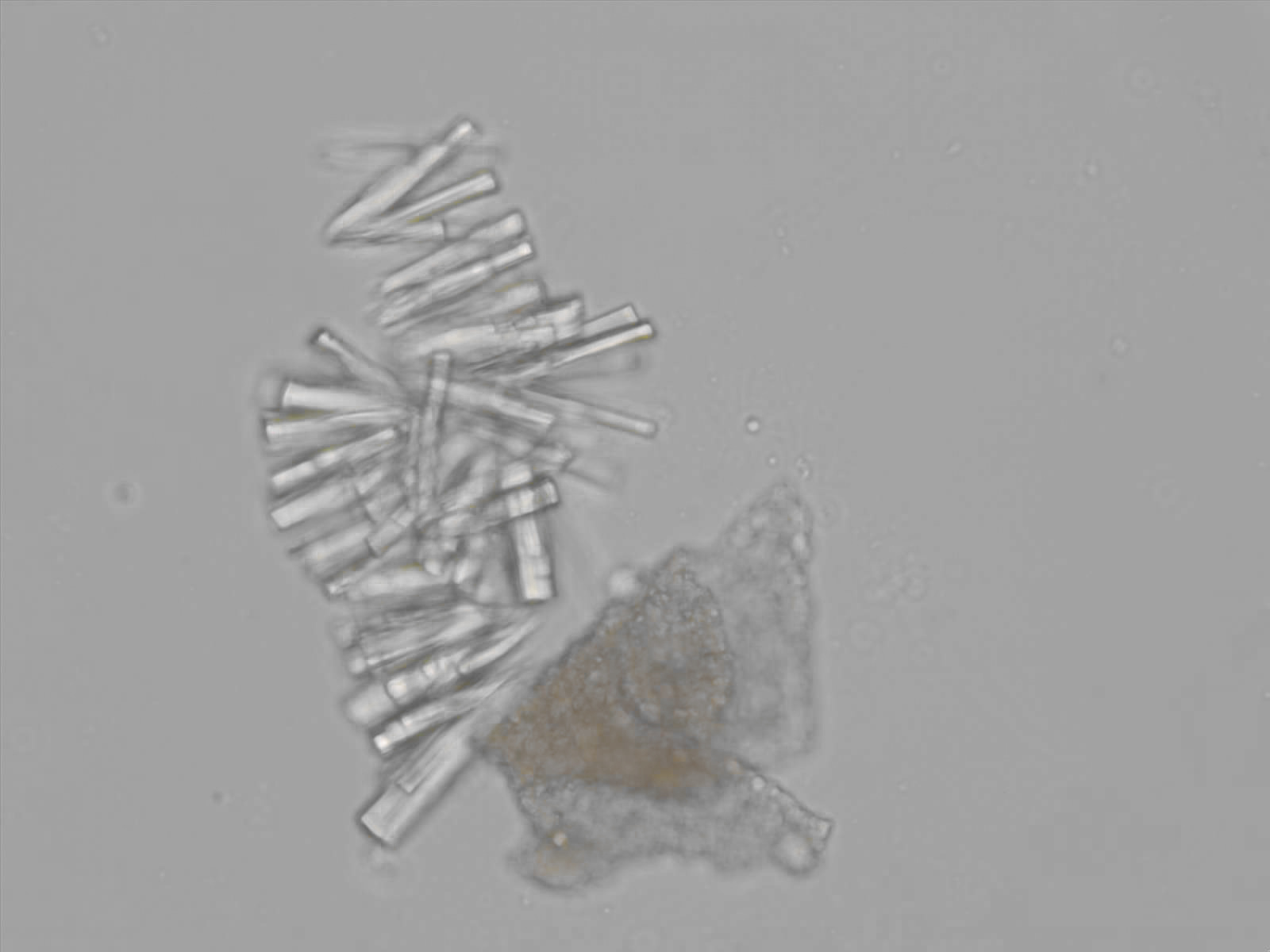
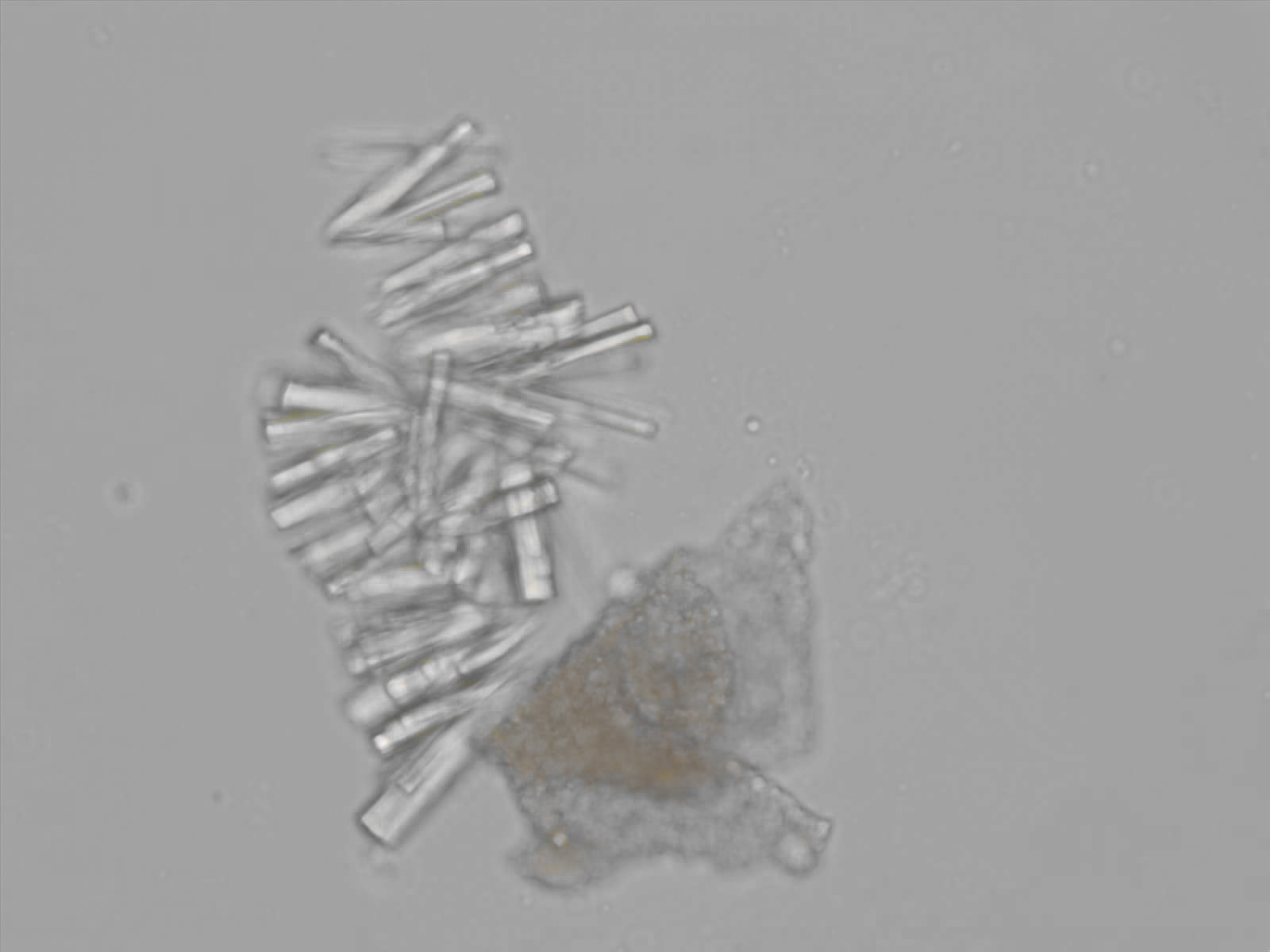
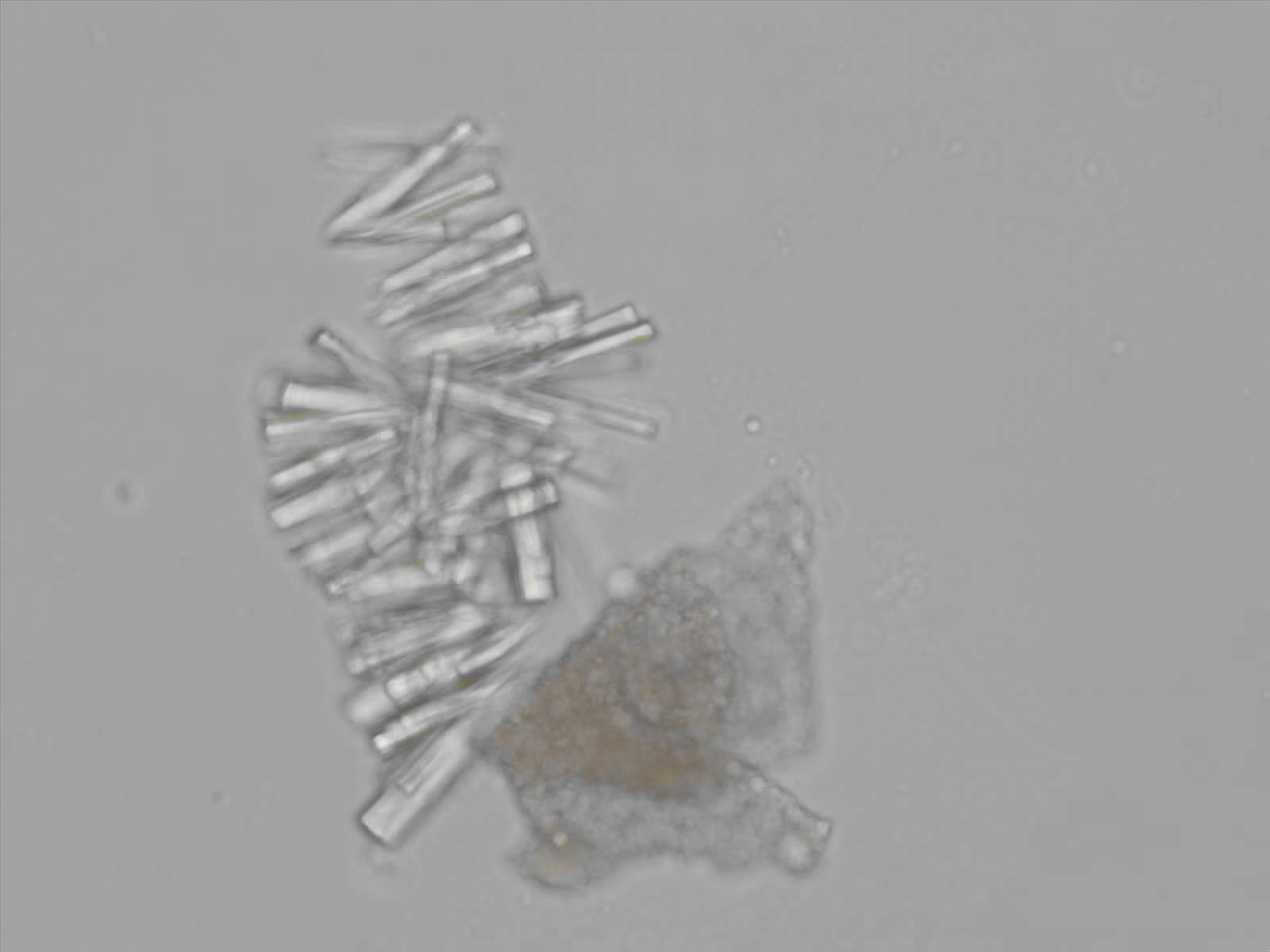
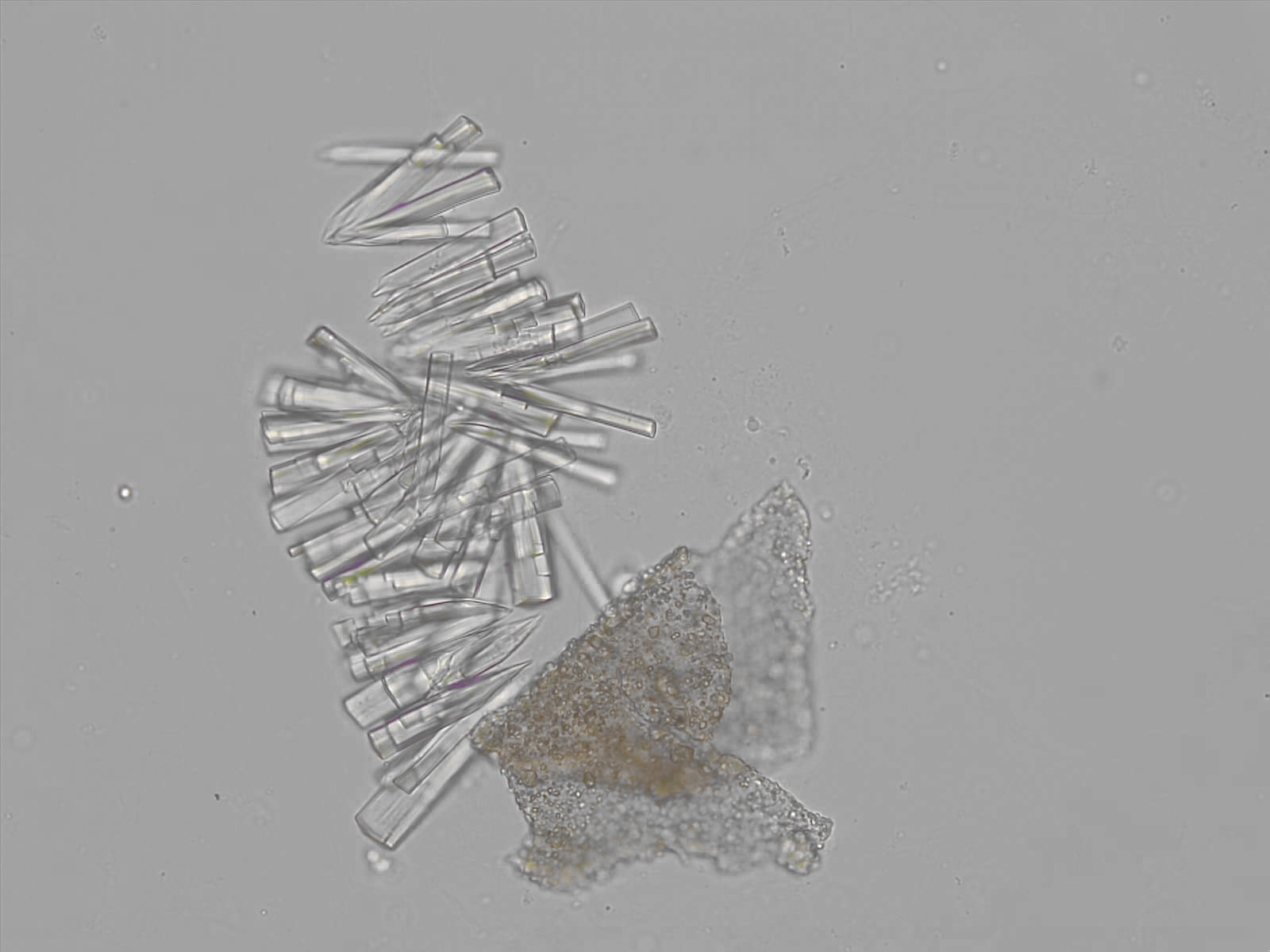
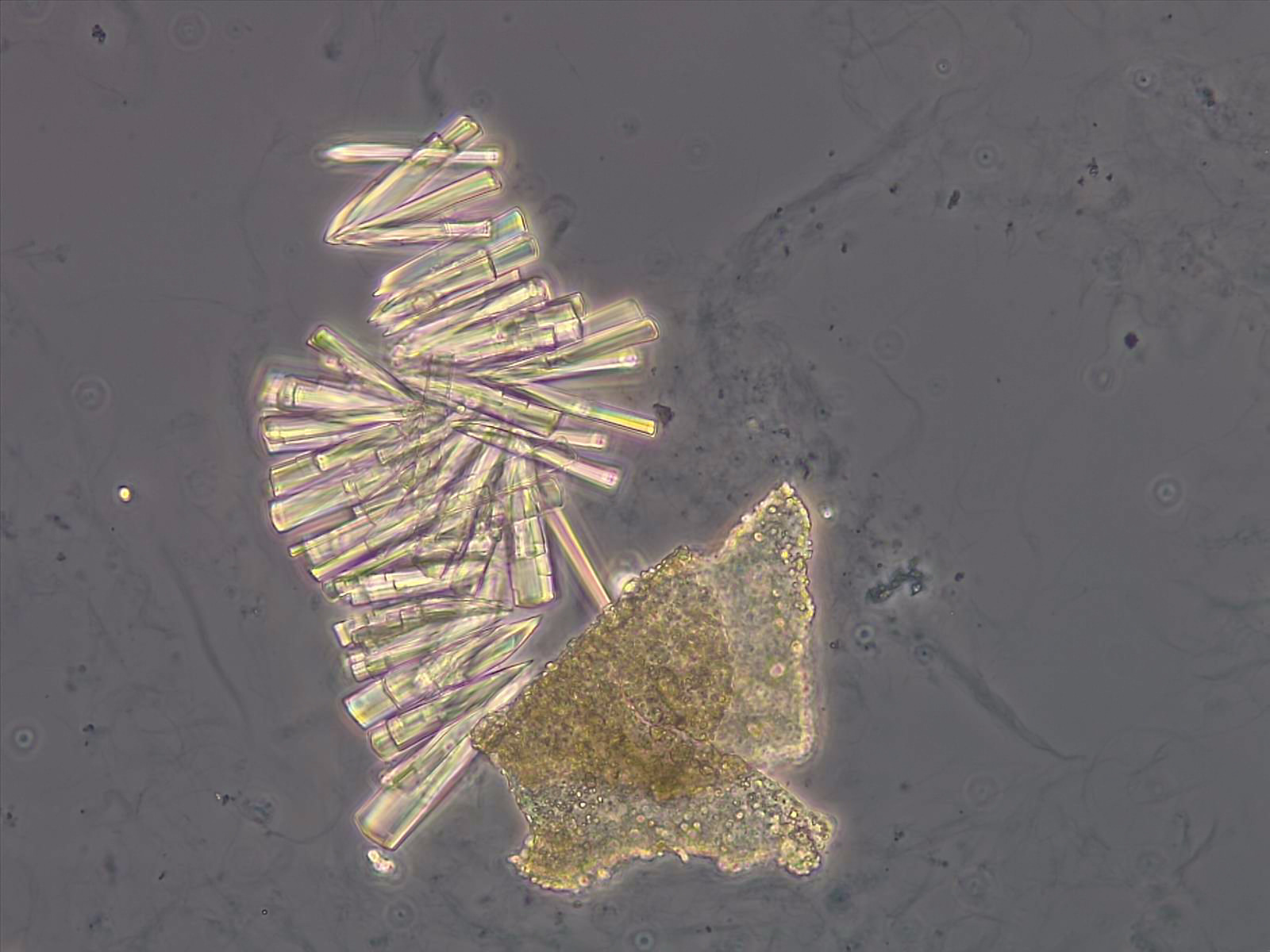
Calcium phosphate - Wedge form
Synonyms: Brushite, CaHPO₄·2H₂O
Calcium phosphate crystals frequently occur in normal urine with an alkaline pH (>7.0), but are also seen in people who form kidney stones. They are colorless and show great morphological variation. The crystals may occur as prismatic shapes, needle or wedge-shaped rods with usually blunt ends, and sometimes as star-shaped clusters (rosettes). These shapes are usually strongly birefringent. In addition, large, flat plates with irregular edges and granular structure can also be found. However, these plates are not birefringent.
Wedge-shaped calcium phosphate crystals may resemble crystals of uric acid, but the distinction can be made on the basis of the urinary pH (acidic in uric acid, basic in calcium phosphate) and the optical behavior under polarized light: uric acid crystals are birefringent, calcium phosphate sheets usually are not.

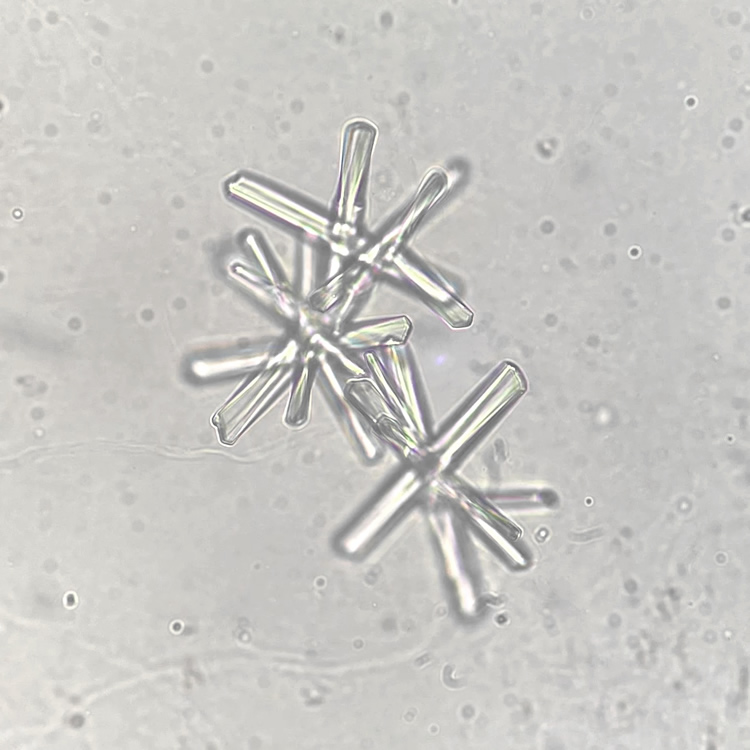
Cross section
Technique
Cross section